Comparative study of allogenic and xenogeneic mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague-Dawley rats
- PMID: 27585525
- PMCID: PMC5009659
- DOI: 10.1186/s13287-016-0386-0
Comparative study of allogenic and xenogeneic mesenchymal stem cells on cisplatin-induced acute kidney injury in Sprague-Dawley rats
Abstract
Background: The paracrine and regenerative activities of mesenchymal stem cells (MSCs) may vary with different stem cell sources. The aim of the present study is to compare the effects of MSCs from different sources on acute kidney injury (AKI) induced by cisplatin and their influence on renal regeneration.
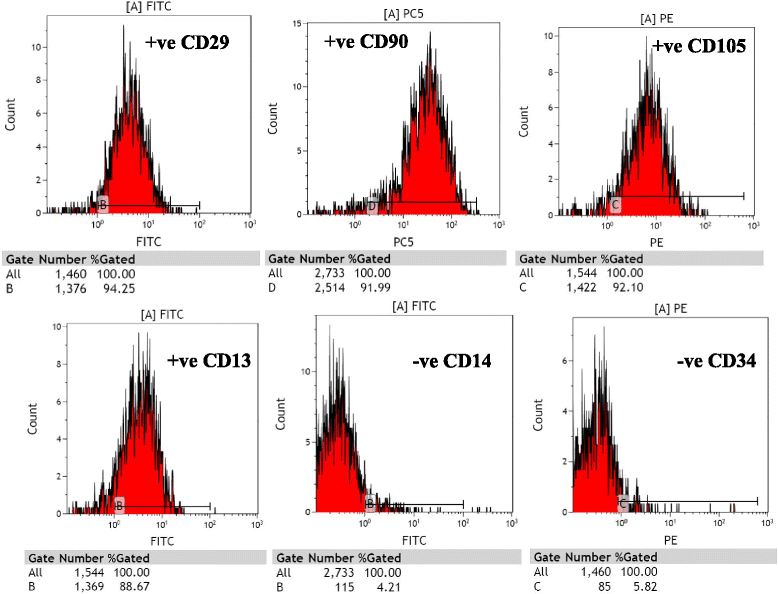
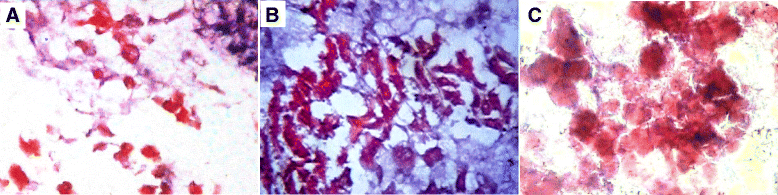
Methods: A single intraperitoneal injection of cisplatin (5 mg/kg) was used to induce AKI in 120 Sprague-Dawley rats. Rats were treated with either rat bone marrow stem cells (rBMSCs), human adipose tissue-derived stem cells (hADSCs), or human amniotic fluid-derived stem cells (hAFSCs). 5 × 10(6) MSCs of different sources were administered through rat tail vein in a single dose, 24 hours after cisplatin injection. Within each group, rats were sacrificed at the 4th, 7th, 11th, and 30th day after cisplatin injection. Serum creatinine, BUN, and renal tissue oxidative stress parameters were measured. Renal tissue was scored histopathologically for evidence of injury, regeneration, and chronicity. Immunohistochemistry was also done using Ki67 for renal proliferative activity evaluation.
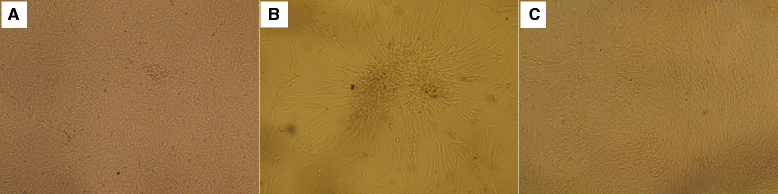
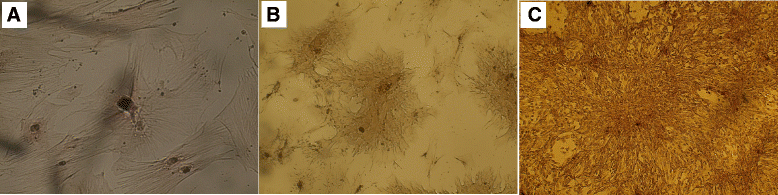
Results: MSCs of the three sources were able to ameliorate cisplatin-induced renal function deterioration and tissue damage. The rat BMSCs-treated group had the lowest serum creatinine by day 30 (0.52 ± 0.06) compared to hADSCs and hAFSCs. All MSC-treated groups had nearly equal antioxidant activity as indicated by the decreased renal tissue malondialdehyde (MDA) and increased reduced glutathione (GSH) level and superoxide dismutase (SOD) activity at different time intervals. Additionally, all MSCs improved injury and regenerative scores. Rat BMSCs had the highest count and earliest proliferative activity in the renal cortex by day 7 as identified by Ki67; while, hAFSCs seem to have the greatest improvement in the regenerative and proliferative activities with a higher count of renal cortex Ki67-positive cells at day 11 and with the least necrotic lesions.
Conclusions: Rat BMSCs, hADSCs, and hAFSCs, in early single IV dose, had a renoprotective effect against cisplatin-induced AKI, and were able to reduce oxidative stress markers. Rat BMSCs had the earliest proliferative activity by day 7; however, hAFSCs seemed to have the greatest improvement in the regenerative activities. Human ADSCs were the least effective in the terms of proliferative and regenerative activities.
Keywords: Adipose-derived; Amniotic fluid-derived; Cisplatin nephrotoxicity; Mesenchymal stem cells.
Figures






Similar articles
-
Efficiency of endovenous versus arterial administration of mesenchymal stem cells for ischemia-reperfusion-induced renal dysfunction in rats.Transplant Proc. 2013 Mar;45(2):503-10. doi: 10.1016/j.transproceed.2012.07.162. Transplant Proc. 2013. PMID: 23498785
-
Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats.Int J Stem Cells. 2016 May 30;9(1):70-8. doi: 10.15283/ijsc.2016.9.1.70. Int J Stem Cells. 2016. PMID: 27426088 Free PMC article.
-
Long Term Study of Protective Mechanisms of Human Adipose Derived Mesenchymal Stem Cells on Cisplatin Induced Kidney injury in Sprague-Dawley Rats.J Stem Cells Regen Med. 2016 May 30;12(1):36-48. doi: 10.46582/jsrm.1201006. eCollection 2016. J Stem Cells Regen Med. 2016. PMID: 27398000 Free PMC article.
-
Regenerative pharmacology for the treatment of acute kidney injury: Skeletal muscle stem/progenitor cells for renal regeneration?Pharmacol Res. 2016 Nov;113(Pt B):802-807. doi: 10.1016/j.phrs.2016.03.014. Epub 2016 Mar 18. Pharmacol Res. 2016. PMID: 27001227 Review.
-
Mesenchymal stem cell therapy in acute kidney injury (AKI): review and perspectives.Rev Assoc Med Bras (1992). 2020 Jan 13;66Suppl 1(Suppl 1):s45-s54. doi: 10.1590/1806-9282.66.S1.45. Rev Assoc Med Bras (1992). 2020. PMID: 31939535 Review.
Cited by
-
hucMSCs Attenuate IBD through Releasing miR148b-5p to Inhibit the Expression of 15-lox-1 in Macrophages.Mediators Inflamm. 2019 May 28;2019:6953963. doi: 10.1155/2019/6953963. eCollection 2019. Mediators Inflamm. 2019. PMID: 31275059 Free PMC article.
-
Concise Reviews: Stem Cells and Kidney Regeneration: An Update.Stem Cells Transl Med. 2019 Jan;8(1):82-92. doi: 10.1002/sctm.18-0115. Epub 2018 Oct 9. Stem Cells Transl Med. 2019. PMID: 30302937 Free PMC article. Review.
-
Activated SOX9+ renal epithelial cells promote kidney repair through secreting factors.Cell Prolif. 2023 Apr;56(4):e13394. doi: 10.1111/cpr.13394. Epub 2023 Jan 4. Cell Prolif. 2023. PMID: 36601693 Free PMC article.
-
Stem/Stromal Cells for Treatment of Kidney Injuries With Focus on Preclinical Models.Front Med (Lausanne). 2018 Jun 15;5:179. doi: 10.3389/fmed.2018.00179. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29963554 Free PMC article. Review.
-
Safety and efficacy of umbilical cord tissue-derived mesenchymal stem cells in the treatment of patients with aging frailty: a phase I/II randomized, double-blind, placebo-controlled study.Stem Cell Res Ther. 2024 Apr 29;15(1):122. doi: 10.1186/s13287-024-03707-2. Stem Cell Res Ther. 2024. PMID: 38679727 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

