Immune phenotypes predict survival in patients with glioblastoma multiforme
- PMID: 27585656
- PMCID: PMC5009501
- DOI: 10.1186/s13045-016-0272-3
Immune phenotypes predict survival in patients with glioblastoma multiforme
Abstract
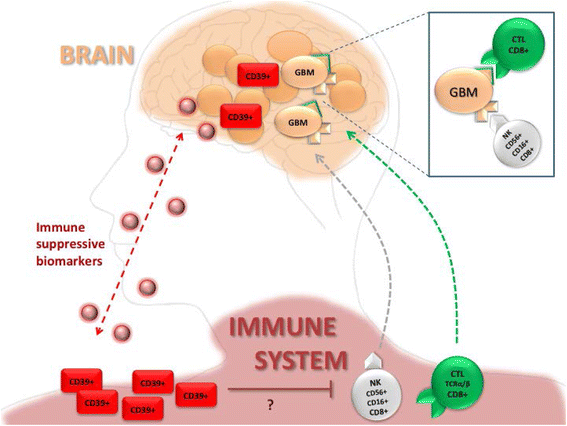
Background: Glioblastoma multiforme (GBM), a common primary malignant brain tumor, rarely disseminates beyond the central nervous system and has a very bad prognosis. The current study aimed at the analysis of immunological control in individual patients with GBM.
Methods: Immune phenotypes and plasma biomarkers of GBM patients were determined at the time of diagnosis using flow cytometry and ELISA, respectively.
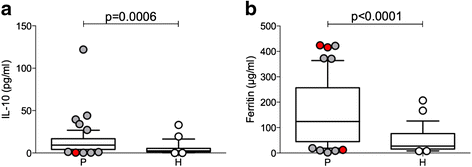
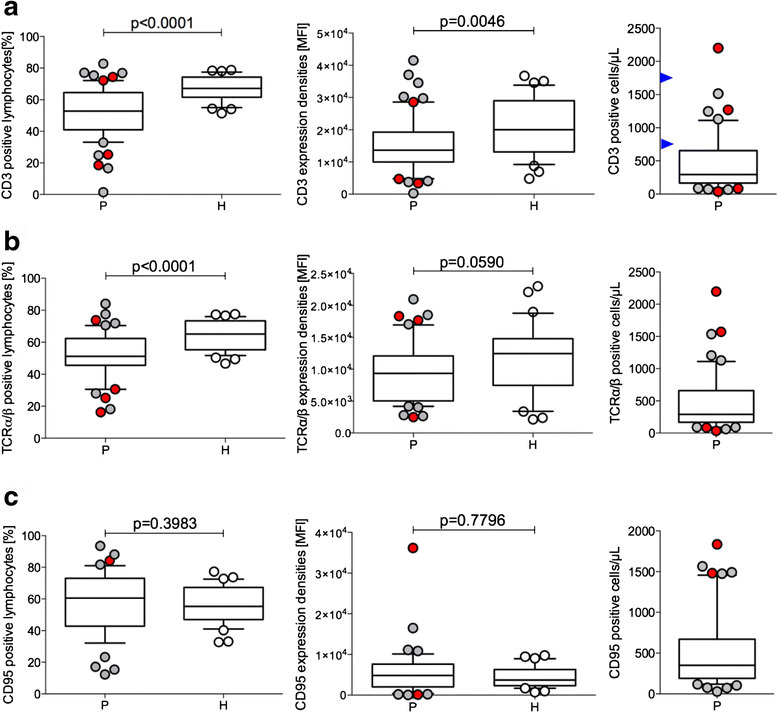
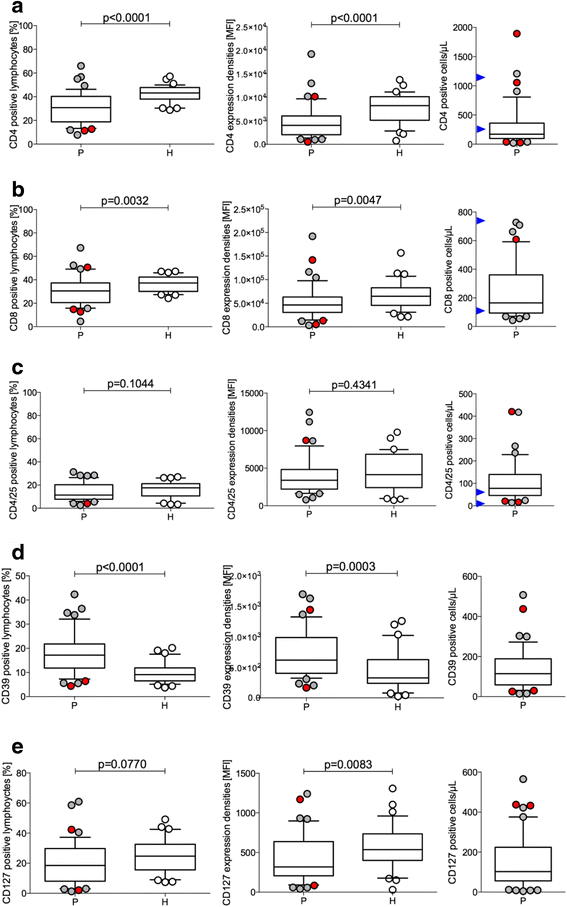
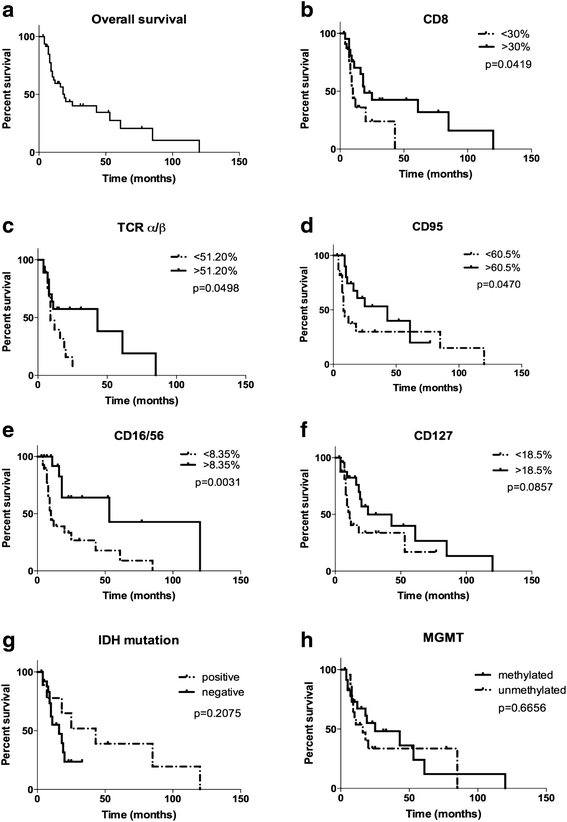
Results: Using descriptive statistics, we found that immune anomalies were distinct in individual patients. Defined marker profiles proved highly relevant for survival. A remarkable relation between activated NK cells and improved survival in GBM patients was in contrast to increased CD39 and IL-10 in patients with a detrimental course and very short survival. Recursive partitioning analysis (RPA) and Cox proportional hazards models substantiated the relevance of absolute numbers of CD8 cells and low numbers of CD39 cells for better survival.
Conclusions: Defined alterations of the immune system may guide the course of disease in patients with GBM and may be prognostically valuable for longitudinal studies or can be applied for immune intervention.
Keywords: CD39-ectonucleotidase; CD8+ lymphocytes; Glioblastoma multiforme; NK cells; Recursive partitioning analysis; Survival.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

