Smad4 controls bone homeostasis through regulation of osteoblast/osteocyte viability
- PMID: 27585718
- PMCID: PMC5050296
- DOI: 10.1038/emm.2016.75
Smad4 controls bone homeostasis through regulation of osteoblast/osteocyte viability
Abstract
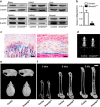
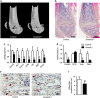
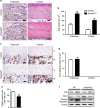
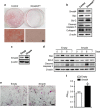
Regulation of osteoblast and osteocyte viability is essential for bone homeostasis. Smad4, a major transducer of bone morphogenetic protein and transforming growth factor-β signaling pathways, regulates apoptosis in various cell types through a mitochondrial pathway. However, it remains poorly understood whether Smad4 is necessary for the regulation of osteoblast and osteocyte viability. In this study, we analyzed Smad4Δ(Os) mice, in which Smad4 was subjected to tissue-specific disruption under the control of the 2.3-kb Col1a1 promoter, to understand the functional significance of Smad4 in regulating osteoblast/osteocyte viability during bone formation and remodeling. Smad4Δ(Os) mice showed a significant increase in osteoblast number and osteocyte density in the trabecular and cortical regions of the femur, whereas osteoclast activity was significantly decreased. The proliferation of osteoblasts/osteocytes did not alter, as shown by measuring 5'-bromo-2'deoxyuridine incorporation. By contrast, the percentage of TUNEL-positive cells decreased, together with a decrease in the Bax/Bcl-2 ratio and in the proteolytic cleavage of caspase 3, in Smad4Δ(Os) mice. Apoptosis in isolated calvaria cells from Smad4Δ(Os) mice decreased after differentiation, which was consistent with the results of the TUNEL assay and western blotting in Smad4Δ(Os) mice. Conversely, osteoblast cells overexpressing Smad4 showed increased apoptosis. In an apoptosis induction model of Smad4Δ(Os) mice, osteoblasts/osteocytes were more resistant to apoptosis than were control cells, and, consequently, bone remodeling was attenuated. These findings indicate that Smad4 has a significant role in regulating osteoblast/osteocyte viability and therefore controls bone homeostasis.
Figures
Similar articles
-
Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure.J Bone Miner Res. 2004 Sep;19(9):1403-10. doi: 10.1359/JBMR.040516. Epub 2004 Jun 1. J Bone Miner Res. 2004. PMID: 15312240
-
Maturation of cortical bone suppresses periosteal osteoprogenitor proliferation in a paracrine manner.J Mol Histol. 2016 Oct;47(5):445-53. doi: 10.1007/s10735-016-9686-z. Epub 2016 Jul 9. J Mol Histol. 2016. PMID: 27394426
-
Osteoblast and osteocyte apoptosis associated with androgen action in bone: requirement of increased Bax/Bcl-2 ratio.Bone. 2006 May;38(5):637-51. doi: 10.1016/j.bone.2005.10.029. Epub 2006 Jan 18. Bone. 2006. PMID: 16413235
-
Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability.Bone. 2011 Jul;49(1):50-5. doi: 10.1016/j.bone.2010.08.008. Epub 2010 Aug 18. Bone. 2011. PMID: 20727997 Free PMC article. Review.
-
Osteocyte Dysfunction in Joint Homeostasis and Osteoarthritis.Int J Mol Sci. 2021 Jun 17;22(12):6522. doi: 10.3390/ijms22126522. Int J Mol Sci. 2021. PMID: 34204587 Free PMC article. Review.
Cited by
-
YAP/TAZ in Bone and Cartilage Biology.Front Cell Dev Biol. 2022 Jan 4;9:788773. doi: 10.3389/fcell.2021.788773. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35059398 Free PMC article. Review.
-
SMAD4 contributes to chondrocyte and osteocyte development.J Cell Mol Med. 2022 Jan;26(1):1-15. doi: 10.1111/jcmm.17080. Epub 2021 Nov 28. J Cell Mol Med. 2022. PMID: 34841647 Free PMC article. Review.
-
Three-dimensional system enabling the maintenance and directed differentiation of pluripotent stem cells under defined conditions.Sci Adv. 2017 May 12;3(5):e1602875. doi: 10.1126/sciadv.1602875. eCollection 2017 May. Sci Adv. 2017. PMID: 28508073 Free PMC article.
-
Fluid shear stress-induced down-regulation of miR-146a-5p inhibits osteoblast apoptosis via targeting SMAD4.Physiol Res. 2022 Dec 16;71(6):835-848. doi: 10.33549/physiolres.934922. Epub 2022 Oct 13. Physiol Res. 2022. PMID: 36281726 Free PMC article.
-
Comparison In Vitro Study on the Interface between Skin and Bone Cell Cultures and Microporous Titanium Samples Manufactured with 3D Printing Technology Versus Sintered Samples.Nanomaterials (Basel). 2024 Sep 12;14(18):1484. doi: 10.3390/nano14181484. Nanomaterials (Basel). 2024. PMID: 39330641 Free PMC article.
References
-
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell 2002; 2: 389–406. - PubMed
-
- Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem 1994; 55: 273–286. - PubMed
-
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet 2003; 4: 638–649. - PubMed
-
- Noble BS, Stevens H, Loveridge N, Reeve J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone 1997; 20: 273–282. - PubMed
-
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 2013; 19: 179–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

