Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome
- PMID: 27586161
- PMCID: PMC5007989
- DOI: 10.1186/s12974-016-0689-1
Cellular investigations with human antibodies associated with the anti-IgLON5 syndrome
Abstract
Background: Antibodies against IgLON5, a neuronal adhesion protein of unknown function, are markers of a novel neurological disorder termed anti-IgLON5 syndrome. The disorder shows a remarkable association with the HLA-DQB1*0501 and HLA-DRB1*1001 alleles, and postmortem studies demonstrate a novel neuronal tauopathy predominantly involving the hypothalamus and tegmentum of the brainstem. The role of IgLON5 antibodies in the pathogenesis of the disease is currently unknown. Here, we have determined the target epitopes of IgLON5 antibodies, the effects of the IgLON5 antibodies in rat hippocampal neurons, and the IgG subclass responsible for these effects.
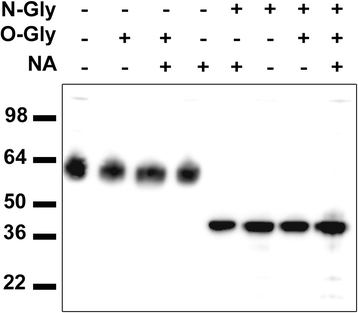
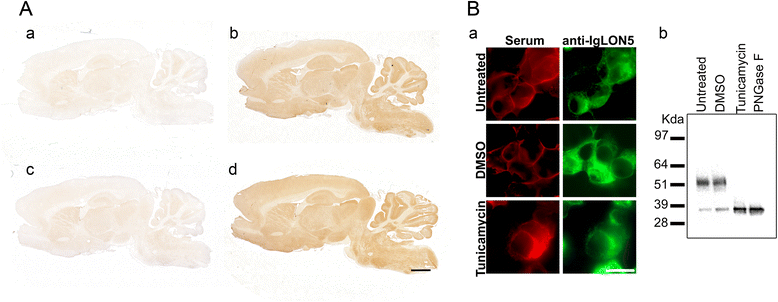
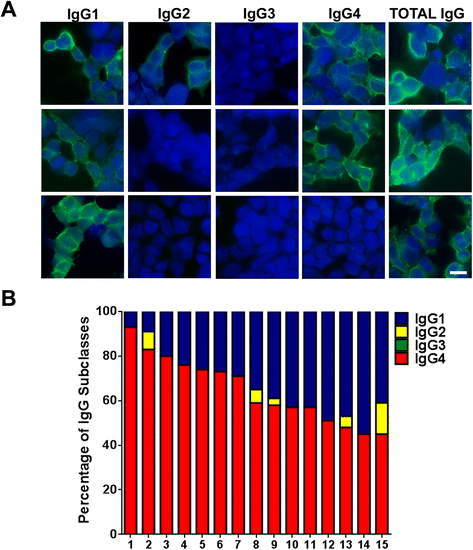
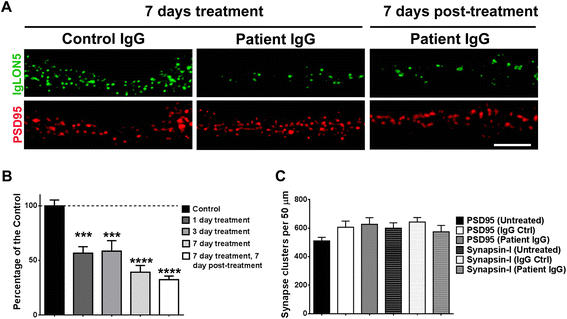
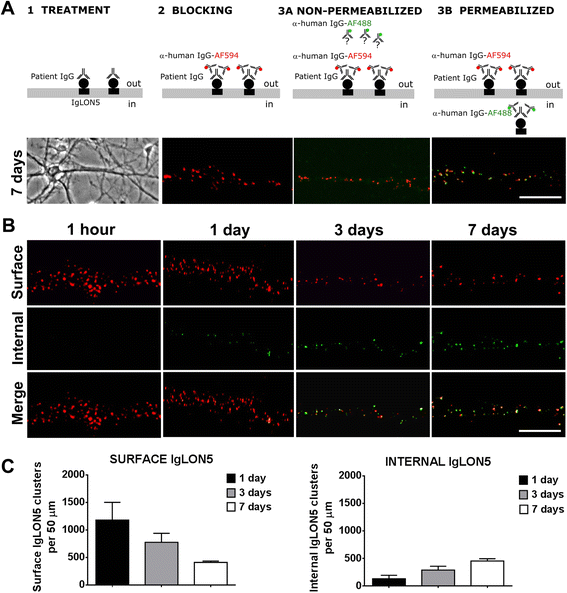
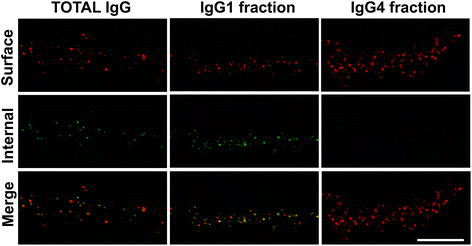
Methods: HEK293 cells expressing several deletion constructs of IgLON5 were used to determine the epitopes recognized by the serum of 15 patients with anti-IgLON5 syndrome. The role of glycosylation in immunogenicity was tested with PNGase F treatment of transfected cells. Dissociated hippocampal neuronal cultures were used to test by immunocytochemistry the effects of total IgG, IgG1, and IgG4 subclasses of IgLON5 antibodies.
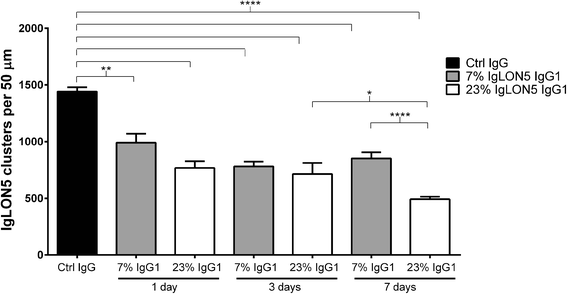
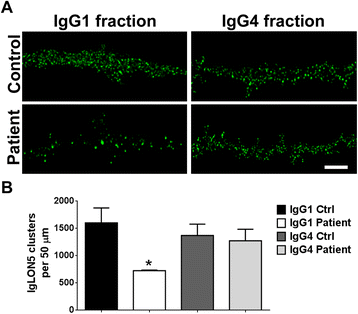
Results: Patients' antibodies reacted with the immunoglobulin-like domain 2 of IgLON5. Glycosylation was not required for immunoreactivity. The predominant subclass of IgLON5 antibodies was IgG4 but all patients also had IgG1. The mean percentage of specific IgLON5 IgG4 and IgG1 of the samples analyzed by flow cytometry was 64 and 33 %, respectively. In cultures of hippocampal neurons, patients' antibodies caused a decrease of cell surface IgLON5 clusters that was not reversed after IgLON5 antibodies were removed from the media. The decrease of surface IgLON5 clusters correlated with the rate of antibody internalization. These effects were observed with purified IgG1 but not with the IgG4 antibodies.
Conclusions: IgLON5 antibodies recognize the immunoglobulin-like domain 2 of the antigen, and the reactivity is not dependent on glycosylation. The effects observed on hippocampal neuronal cultures indicate an irreversible antibody-mediated internalization of surface IgLON5. These effects were mediated by specific IgLON5 IgG1 antibodies and suggest a pathogenic role of these antibodies in the disease.
Keywords: Autoantibodies; IgLON5; Immunoglobulin subclasses; Tauopathy.
Figures

References
-
- Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13:575–86. doi: 10.1016/S1474-4422(14)70051-1. - DOI - PMC - PubMed
-
- Brüggemann N, Wandinger KP, Gaig C, Sprenger A, Junghanns K, Helmchen C, et al. Dystonia, lower limb stiffness, and upward gaze palsy in a patient with IgLON5 antibodies. Mov Disord. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

