A novel method for investigating Burkholderia cenocepacia infections in patients with cystic fibrosis and other chronic diseases of the airways
- PMID: 27586172
- PMCID: PMC5009706
- DOI: 10.1186/s12866-016-0811-7
A novel method for investigating Burkholderia cenocepacia infections in patients with cystic fibrosis and other chronic diseases of the airways
Abstract
Background: Burkholderia cenocepacia is a Gram-negative, opportunistic pathogen that is a cause of morbidity and mortality in patients with cystic fibrosis (CF). Research efforts over the past few decades contributed to our understanding of these infections by identifying virulence factors. However, little is known about how this pathogen adapts to the harsh environment found inside the CF airways, which is characterized by a unique mucus containing high concentrations of inflammatory markers. The current study developed a novel model to further investigate this phenomenon.
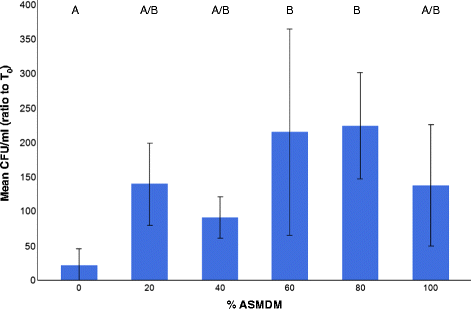
Results: Monolayers of human A549 lung carcinoma cells (HLCCs) were exposed to a mixture of artificial CF sputum medium (ASMDM) in tissue culture growth medium, and subsequently infected with B. cenocepacia K56-2 for 24 h. The data showed that this model supported B. cenocepacia growth. In addition, consistent with similar studies using current models such as CF airway tissue samples, HLCC viability was reduced by more than 70 % when grown in 60 % ASMDM and infected with B. cenocepacia compared to mock-infected controls and medium alone. Furthermore, the amount of B. cenocepacia cells associated with the HLCC monolayer was more than 10 times greater in 60 % ASMDM when compared to medium controls.
Conclusions: These findings suggest that HLCC monolayers in 60 % ASMDM serve as a valid alternative to study B. cenocepacia infections in patients with CF, and possibly other chronic diseases of the airways. Furthermore, the results obtained in this study suggest an important role for CF sputum in B. cenocepacia pathogenesis.
Keywords: Burkholderia; CF sputum; Model; PCD sputum; Tissue culture; cenocepacia.
Figures







Similar articles
-
Synthetic Cystic Fibrosis Sputum Medium Regulates Flagellar Biosynthesis through the flhF Gene in Burkholderia cenocepacia.Front Cell Infect Microbiol. 2016 Jun 14;6:65. doi: 10.3389/fcimb.2016.00065. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27379216 Free PMC article.
-
Belfast Agar-a simple laboratory medium to separate Pseudomonas aeruginosa from pan-resistant Burkholderia cenocepacia isolated from the sputum of patients with cystic fibrosis (CF).Br J Biomed Sci. 2018 Apr;75(2):101-103. doi: 10.1080/09674845.2017.1411018. Epub 2018 Mar 7. Br J Biomed Sci. 2018. PMID: 29513134 No abstract available.
-
Activation of MMP-9 by human lung epithelial cells in response to the cystic fibrosis-associated pathogen Burkholderia cenocepacia reduced wound healing in vitro.Am J Physiol Lung Cell Mol Physiol. 2011 Oct;301(4):L575-86. doi: 10.1152/ajplung.00226.2010. Epub 2011 Jul 8. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21743026
-
Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence.Clin Microbiol Infect. 2010 Jul;16(7):821-30. doi: 10.1111/j.1469-0691.2010.03237.x. Clin Microbiol Infect. 2010. PMID: 20880411 Review.
-
Host evasion by Burkholderia cenocepacia.Front Cell Infect Microbiol. 2012 Jan 11;1:25. doi: 10.3389/fcimb.2011.00025. eCollection 2011. Front Cell Infect Microbiol. 2012. PMID: 22919590 Free PMC article. Review.
Cited by
-
Phage-antibiotic synergy reduces Burkholderia cenocepacia population.BMC Microbiol. 2023 Jan 5;23(1):2. doi: 10.1186/s12866-022-02738-0. BMC Microbiol. 2023. PMID: 36600213 Free PMC article.
-
The Use of Artificial Sputum Media to Enhance Investigation and Subsequent Treatment of Cystic Fibrosis Bacterial Infections.Microorganisms. 2022 Jun 22;10(7):1269. doi: 10.3390/microorganisms10071269. Microorganisms. 2022. PMID: 35888988 Free PMC article. Review.
-
The Efficacy of an N-Acetylcysteine-Antibiotic Combination Therapy on Achromobacter xylosoxidans in a Cystic Fibrosis Sputum/Lung Cell Model.Biomedicines. 2022 Nov 10;10(11):2886. doi: 10.3390/biomedicines10112886. Biomedicines. 2022. PMID: 36359406 Free PMC article.
-
Activity of antibiotics against Burkholderia cepacia complex in artificial sputum medium.J Antimicrob Chemother. 2024 Nov 4;79(11):2867-2876. doi: 10.1093/jac/dkae299. J Antimicrob Chemother. 2024. PMID: 39224940 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

