Nestin(+) cells direct inflammatory cell migration in atherosclerosis
- PMID: 27586429
- PMCID: PMC5025806
- DOI: 10.1038/ncomms12706
Nestin(+) cells direct inflammatory cell migration in atherosclerosis
Abstract
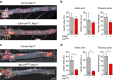
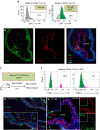
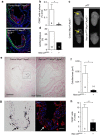
Atherosclerosis is a leading death cause. Endothelial and smooth muscle cells participate in atherogenesis, but it is unclear whether other mesenchymal cells contribute to this process. Bone marrow (BM) nestin(+) cells cooperate with endothelial cells in directing monocyte egress to bloodstream in response to infections. However, it remains unknown whether nestin(+) cells regulate inflammatory cells in chronic inflammatory diseases, such as atherosclerosis. Here, we show that nestin(+) cells direct inflammatory cell migration during chronic inflammation. In Apolipoprotein E (ApoE) knockout mice fed with high-fat diet, BM nestin(+) cells regulate the egress of inflammatory monocytes and neutrophils. In the aorta, nestin(+) stromal cells increase ∼30 times and contribute to the atheroma plaque. Mcp1 deletion in nestin(+) cells-but not in endothelial cells only- increases circulating inflammatory cells, but decreases their aortic infiltration, delaying atheroma plaque formation and aortic valve calcification. Therefore, nestin expression marks cells that regulate inflammatory cell migration during atherosclerosis.
Figures



References
-
- Cybulsky M. I. & Gimbrone M. A. Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251, 788–791 (1991). - PubMed
-
- Zhang S. H., Reddick R. L., Piedrahita J. A. & Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 258, 468–471 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

