Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1
- PMID: 27586459
- PMCID: PMC5025761
- DOI: 10.1038/ncomms12565
Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1
Abstract
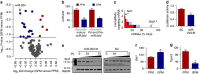
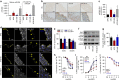
Gut microbiota promotes atherosclerosis, and vascular endothelial dysfunction, signalled by impaired endothelium-dependent vasorelaxation, is an early marker of atherosclerosis. Here we show that vascular microRNA-204 (miR-204) expression is remotely regulated by the microbiome, and impairs endothelial function by targeting the Sirtuin1 lysine deacetylase (Sirt1). MiR-204 is downregulated, while Sirt1 is upregulated, in aortas of germ-free mice. Suppression of gut microbiome with broad-spectrum antibiotics decreases miR-204, increases Sirt1 and bioavailable vascular nitric oxide, and improves endothelium-dependent vasorelaxation in mouse aortas. Antibiotics curtail aortic miR-204 upregulation, and rescue decline of aortic Sirt1 and endothelium-dependent vasorelaxation, triggered by high-fat diet feeding. Improvement of endothelium-dependent vasorelaxation by antibiotics is lost in mice lacking endothelial Sirt1. Systemic antagonism of miR-204 rescues impaired endothelium-dependent vasorelaxation and vascular Sirt1, and decreases vascular inflammation induced by high-fat diet. These findings reveal a gut microbe-vascular microRNA-Sirtuin1 nexus that leads to endothelial dysfunction.
Figures



References
-
- Qin J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

