Replication Timing of Human Telomeres is Conserved during Immortalization and Influenced by Respective Subtelomeres
- PMID: 27587191
- PMCID: PMC5009427
- DOI: 10.1038/srep32510
Replication Timing of Human Telomeres is Conserved during Immortalization and Influenced by Respective Subtelomeres
Abstract
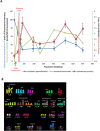
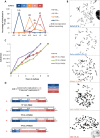
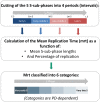
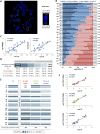
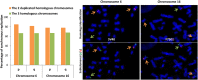
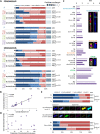
Telomeres are specific structures that protect chromosome ends and act as a biological clock, preventing normal cells from replicating indefinitely. Mammalian telomeres are replicated throughout S-phase in a predetermined order. However, the mechanism of this regulation is still unknown. We wished to investigate this phenomenon under physiological conditions in a changing environment, such as the immortalization process to better understand the mechanism for its control. We thus examined the timing of human telomere replication in normal and SV40 immortalized cells, which are cytogenetically very similar to cancer cells. We found that the timing of telomere replication was globally conserved under different conditions during the immortalization process. The timing of telomere replication was conserved despite changes in telomere length due to endogenous telomerase reactivation, in duplicated homologous chromosomes, and in rearranged chromosomes. Importantly, translocated telomeres, possessing their initial subtelomere, retained the replication timing of their homolog, independently of the proportion of the translocated arm, even when the remaining flanking DNA is restricted to its subtelomere, the closest chromosome-specific sequences (inferior to 500 kb). Our observations support the notion that subtelomere regions strongly influence the replication timing of the associated telomere.
Figures
Similar articles
-
Replication timing of human telomeres is chromosome arm-specific, influenced by subtelomeric structures and connected to nuclear localization.PLoS Genet. 2010 Apr 22;6(4):e1000920. doi: 10.1371/journal.pgen.1000920. PLoS Genet. 2010. PMID: 20421929 Free PMC article.
-
Telomere dynamics, end-to-end fusions and telomerase activation during the human fibroblast immortalization process.Oncogene. 1999 Jul 22;18(29):4211-23. doi: 10.1038/sj.onc.1202797. Oncogene. 1999. PMID: 10435634
-
Asynchronous replication timing of telomeres at opposite arms of mammalian chromosomes.Proc Natl Acad Sci U S A. 2004 Aug 31;101(35):12928-33. doi: 10.1073/pnas.0404106101. Epub 2004 Aug 20. Proc Natl Acad Sci U S A. 2004. PMID: 15322275 Free PMC article.
-
Telomere length homeostasis.Chromosoma. 2006 Dec;115(6):413-25. doi: 10.1007/s00412-006-0067-3. Epub 2006 Jun 2. Chromosoma. 2006. PMID: 16741708 Review.
-
Sizing the ends: normal length of human telomeres.Ann Anat. 2010 Sep 20;192(5):284-91. doi: 10.1016/j.aanat.2010.07.005. Epub 2010 Aug 6. Ann Anat. 2010. PMID: 20732797 Review.
Cited by
-
CTCF driven TERRA transcription facilitates completion of telomere DNA replication.Nat Commun. 2017 Dec 13;8(1):2114. doi: 10.1038/s41467-017-02212-w. Nat Commun. 2017. PMID: 29235471 Free PMC article.
-
Bi-allelic MCM10 variants associated with immune dysfunction and cardiomyopathy cause telomere shortening.Nat Commun. 2021 Mar 12;12(1):1626. doi: 10.1038/s41467-021-21878-x. Nat Commun. 2021. PMID: 33712616 Free PMC article.
-
Bring It to an End: Does Telomeres Size Matter?Cells. 2019 Jan 8;8(1):30. doi: 10.3390/cells8010030. Cells. 2019. PMID: 30626097 Free PMC article. Review.
-
Advancements in extracellular vesicle targeted therapies for rheumatoid arthritis: insights into cellular origins, current perspectives, and emerging challenges.Stem Cell Res Ther. 2024 Sep 4;15(1):276. doi: 10.1186/s13287-024-03887-x. Stem Cell Res Ther. 2024. PMID: 39227964 Free PMC article. Review.
-
DNA Replication Origins and Fork Progression at Mammalian Telomeres.Genes (Basel). 2017 Mar 28;8(4):112. doi: 10.3390/genes8040112. Genes (Basel). 2017. PMID: 28350373 Free PMC article. Review.
References
-
- Blackburn E. H. Switching and signaling at the telomere. Cell. 106, 661–673 (2001). - PubMed
-
- Griffith J. D. et al. Mammalian telomeres end in a large duplex loop. Cell. 97, 503–514 (1999). - PubMed
-
- Wellinger R. J.& Sen D. The DNA structures at the ends of eukaryotic chromosomes. Eur J Cancer. 33, 735–749 (1997). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

