Molecular mechanisms of Ebola pathogenesis
- PMID: 27587404
- PMCID: PMC6608070
- DOI: 10.1189/jlb.4RI0316-099RR
Molecular mechanisms of Ebola pathogenesis
Abstract
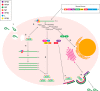
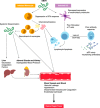
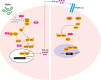
Ebola viruses (EBOVs) and Marburg viruses (MARVs) are among the deadliest human viruses, as highlighted by the recent and widespread Ebola virus outbreak in West Africa, which was the largest and longest epidemic of Ebola virus disease (EVD) in history, resulting in significant loss of life and disruptions across multiple continents. Although the number of cases has nearly reached its nadir, a recent cluster of 5 cases in Guinea on March 17, 2016, has extended the enhanced surveillance period to June 15, 2016. New, enhanced 90-d surveillance windows replaced the 42-d surveillance window to ensure the rapid detection of new cases that may arise from a missed transmission chain, reintroduction from an animal reservoir, or more important, reemergence of the virus that has persisted in an EVD survivor. In this review, we summarize our current understanding of EBOV pathogenesis, describe vaccine and therapeutic candidates in clinical trials, and discuss mechanisms of viral persistence and long-term health sequelae for EVD survivors.
Keywords: Ebola survivors; Ebola vaccines; antivirals; immunoevasion; viral hemorrhagic fever.
© Society for Leukocyte Biology.
Figures
References
-
- Feldmann, H. , Slenczka, W. , Klenk, H. D. (1996) Emerging and reemerging of filoviruses. Arch. Virol. Suppl. 11, 77–100. - PubMed
-
- Slenczka, W. , Klenk, H. D. (2007) Forty years of Marburg virus. J. Infect. Dis. 196 (Suppl 2), S131–S135. - PubMed
-
- Siegert, R. , Shu, H. L. , Slenczka, W. , Peters, D. , Müller, G. (1967) On the etiology of an unknown human infection originating from monkeys [in German]. Dtsch. Med. Wochenschr. 92, 2341–2343. - PubMed
-
- Towner, J. S. , Khristova, M. L. , Sealy, T. K. , Vincent, M. J. , Erickson, B. R. , Bawiec, D. A. , Hartman, A. L. , Comer, J. A. , Zaki, S. R. , Ströher, U. , Gomes da Silva, F. , del Castillo, F. , Rollin, P. E. , Ksiazek, T. G. , Nichol, S. T. (2006) Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 80, 6497–6516. - PMC - PubMed
-
- Bausch, D. G. , Nichol, S. T. , Muyembe‐Tamfum, J. J. , Borchert, M. , Rollin, P. E. , Sleurs, H. , Campbell, P. , Tshioko, F. K. , Roth, C. , Colebunders, R. , Pirard, P. , Mardel, S. , Olinda, L. A. , Zeller, H. , Tshomba, A. , Kulidri, A. , Libande, M. L. , Mulangu, S. , Formenty, P. , Grein, T. , Leirs, H. , Braack, L. , Ksiazek, T. , Zaki, S. , Bowen, M. D. , Smit, S. B. , Leman, P. A. , Burt, F. J. , Kemp, A. , Swanepoel, R. ; International Scientific and Technical Committee for Marburg Hemorrhagic Fever Control in the Democratic Republic of the Congo . (2006) Marburg hemorrhagic fever associated with multiple genetic lineages of virus. N. Engl. J. Med. 355, 909–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

