Percutaneous Device Closure of Paravalvular Leak: Combined Experience From the United Kingdom and Ireland
- PMID: 27587432
- PMCID: PMC6485596
- DOI: 10.1161/CIRCULATIONAHA.116.022684
Percutaneous Device Closure of Paravalvular Leak: Combined Experience From the United Kingdom and Ireland
Erratum in
-
Correction to: Percutaneous Device Closure of Paravalvular Leak: Combined Experience From the United Kingdom and Ireland.Circulation. 2016 Nov 1;134(18):e403. doi: 10.1161/CIR.0000000000000466. Circulation. 2016. PMID: 27799268 No abstract available.
Abstract
Background: Paravalvular leak (PVL) occurs in 5% to 17% of patients following surgical valve replacement. Percutaneous device closure represents an alternative to repeat surgery.
Methods: All UK and Ireland centers undertaking percutaneous PVL closure submitted data to the UK PVL Registry. Data were analyzed for association with death and major adverse cardiovascular events (MACE) at follow-up.
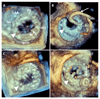
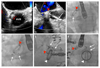
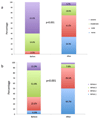
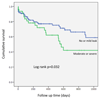
Results: Three hundred eight PVL closure procedures were attempted in 259 patients in 20 centers (2004-2015). Patient age was 67±13 years; 28% were female. The main indications for closure were heart failure (80%) and hemolysis (16%). Devices were successfully implanted in 91% of patients, via radial (7%), femoral arterial (52%), femoral venous (33%), and apical (7%) approaches. Nineteen percent of patients required repeat procedures. The target valve was mitral (44%), aortic (48%), both (2%), pulmonic (0.4%), or transcatheter aortic valve replacement (5%). Preprocedural leak was severe (61%), moderate (34%), or mild (5.7%) and was multiple in 37%. PVL improved postprocedure (P<0.001) and was none (33.3%), mild (41.4%), moderate (18.6%), or severe (6.7%) at last follow-up. Mean New York Heart Association class improved from 2.7±0.8 preprocedure to 1.6±0.8 (P<0.001) after a median follow-up of 110 (7-452) days. Hospital mortality was 2.9% (elective), 6.8% (in-hospital urgent), and 50% (emergency) (P<0.001). MACE during follow-up included death (16%), valve surgery (6%), late device embolization (0.4%), and new hemolysis requiring transfusion (1.6%). Mitral PVL was associated with higher MACE (hazard ratio [HR], 1.83; P=0.011). Factors independently associated with death were the degree of persisting leak (HR, 2.87; P=0.037), New York Heart Association class (HR, 2.00; P=0.015) at follow-up and baseline creatinine (HR, 8.19; P=0.001). The only factor independently associated with MACE was the degree of persisting leak at follow-up (HR, 3.01; P=0.002).
Conclusion: Percutaneous closure of PVL is an effective procedure that improves PVL severity and symptoms. Severity of persisting leak at follow-up is independently associated with both MACE and death. Percutaneous closure should be considered as an alternative to repeat surgery.
Keywords: catheters; heart failure; paravalvular regurgitation; survival.
© 2016 American Heart Association, Inc.
Figures




References
-
- Herr R, Starr A, Mccord CW, Wood JA. SPECIAL PROBLEMS FOLLOWING VALVE REPLACEMENT: EMBOLUS, LEAK, INFECTION, RED CELL DAMAGE. Ann Thorac Surg. 1965;1:403–415. - PubMed
-
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–1158. - PubMed
-
- Gafoor S, Steinberg DH, Franke J, Bertog S, Vaskelyte L, Hofmann I, Sievert H. Tools and Techniques - Clinical: Paravalvular leak closure. EuroIntervention. 2014;9:1359–1363. - PubMed
-
- Rihal CS, Sorajja P, Booker JD, Hagler DJ, Cabalka AK. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc Interv. 2012;5:121–130. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

