Development and Morphology of the Ventricular Outflow Tracts
- PMID: 27587491
- PMCID: PMC5011314
- DOI: 10.1177/2150135116651114
Development and Morphology of the Ventricular Outflow Tracts
Abstract
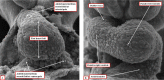
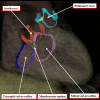
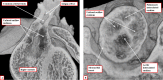
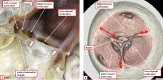
It is customary, at the current time, to consider many, if not most, of the lesions involving the ventricular outflow tract in terms of conotruncal malformations. This reflects the introduction, in the early 1940s, of the terms conus and truncus to describe the components of the developing outflow tract. The definitive outflow tracts in the postnatal heart, however, possess three, rather than two, components. These are the intrapericardial arterial trunks, the arterial roots, and the subvalvar ventricular outflow tracts. Congenital lesions afflicting the arterial roots, however, are not currently considered to be conotruncal malformations. This suggests a lack of logic in the description of cardiac development and its use as a means of categorizing congenital malformations. It is our belief that the developing outflow tract, like the postnatal outflow tracts, can readily be described in tripartite fashion, with its distal, intermediate, and proximal components forming the primordiums of the postnatal parts. In this review, we present evidence obtained from developing mice and human hearts to substantiate this notion. We show that the outflow tract, initially with a common lumen, is divided into its aortic and pulmonary components by a combination of an aortopulmonary septum derived from the dorsal wall of the aortic sac and outflow tract cushions that spiral through its intermediate and proximal components. These embryonic septal structures, however, subsequently lose their septal functions as the outflow tracts develop their own discrete walls. We then compare the developmental findings with the anatomic arrangements seen postnatally in the normal human heart. We show how correlations with the embryologic findings permit logical analysis of the congenital lesions involving the outflow tracts.
Keywords: conotruncal anomalies; conus; episcopic microscopy; multidetector computer tomography; normal anatomy; truncus.
© The Author(s) 2016.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
















References
-
- Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Am J Anat. 1942;71(3):343–370.
-
- Roberts WC. The 2 most common congenital heart diseases. Am J Cardiol. 1984;53(8):1198. - PubMed
-
- Lacour Gayet F, Bove EL, Hraska V, Morell VO, Spray TL. Surgery of Conotruncal Anomalies. Switzerland: Springer International Publishing; 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

