Host cell remodeling by pathogens: the exomembrane system in Plasmodium-infected erythrocytes
- PMID: 27587718
- PMCID: PMC5007283
- DOI: 10.1093/femsre/fuw016
Host cell remodeling by pathogens: the exomembrane system in Plasmodium-infected erythrocytes
Abstract
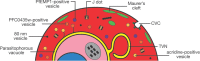
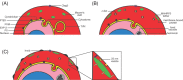
Malaria is caused by infection of erythrocytes by parasites of the genus Plasmodium To survive inside erythrocytes, these parasites induce sweeping changes within the host cell, one of the most dramatic of which is the formation of multiple membranous compartments, collectively referred to as the exomembrane system. As an uninfected mammalian erythrocyte is devoid of internal membranes, the parasite must be the force and the source behind the formation of these compartments. Even though the first evidence of the presence these of internal compartments was obtained over a century ago, their functions remain mostly unclear, and in some cases completely unknown, and the mechanisms underlying their formation are still mysterious. In this review, we provide an overview of the different parts of the exomembrane system, describing the parasitophorous vacuole, the tubovesicular network, Maurer's clefts, the caveola-vesicle complex, J dots and other mobile compartments, and the small vesicles that have been observed in Plasmodium-infected cells. Finally, we combine the data into a simplified view of the exomembrane system and its relation to the alterations of the host erythrocyte.
Keywords: exomembrane system; host–parasite interaction; malaria; pathogenesis; plasmodium.
© FEMS 2016.
Figures

References
-
- Abkarian M, Massiera G, Berry L, et al. A novel mechanism for egress of malarial parasites from red blood cells. Blood. 2011;117:4118–24. - PubMed
-
- Abu Bakar N, Klonis N, Hanssen E, et al. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–50. - PubMed
-
- Adisa A, Albano FR, Reeder J, et al. Evidence for a role for a Plasmodium falciparum homologue of Sec 31p in the export of proteins to the surface of malaria parasite-infected erythrocytes. J Cell Sci. 2001;114:3377–86. - PubMed
-
- Adisa A, Frankland S, Rug M, et al. Re-assessing the locations of components of the classical vesicle-mediated trafficking machinery in transfected Plasmodium falciparum. Int J Parasitol. 2007;37:1127–41. - PubMed
-
- Adisa A, Rug M, Foley M, et al. Characterisation of a delta-COP homologue in the malaria parasite, Plasmodium falciparum. Mol Biochem Parasit. 2002;123:11–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

