Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness
- PMID: 27587721
- PMCID: PMC5013887
- DOI: 10.1093/aje/kww064
Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness
Abstract
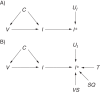
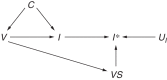
Influenza viruses undergo frequent antigenic changes. As a result, the viruses circulating change within and between seasons, and the composition of the influenza vaccine is updated annually. Thus, estimation of the vaccine's effectiveness is not constant across seasons. In order to provide annual estimates of the influenza vaccine's effectiveness, health departments have increasingly adopted the "test-negative design," using enhanced data from routine surveillance systems. In this design, patients presenting to participating general practitioners with influenza-like illness are swabbed for laboratory testing; those testing positive for influenza virus are defined as cases, and those testing negative form the comparison group. Data on patients' vaccination histories and confounder profiles are also collected. Vaccine effectiveness is estimated from the odds ratio comparing the odds of testing positive for influenza among vaccinated patients and unvaccinated patients, adjusting for confounders. The test-negative design is purported to reduce bias associated with confounding by health-care-seeking behavior and misclassification of cases. In this paper, we use directed acyclic graphs to characterize potential biases in studies of influenza vaccine effectiveness using the test-negative design. We show how studies using this design can avoid or minimize bias and where bias may be introduced with particular study design variations.
Keywords: causal inference; directed acyclic graphs; epidemiologic methods; influenza; observational studies; test-negative study design; vaccine effectiveness.
© The Author 2016. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






Comment in
-
Invited Commentary: Beware the Test-Negative Design.Am J Epidemiol. 2016 Sep 1;184(5):354-6. doi: 10.1093/aje/kww063. Am J Epidemiol. 2016. PMID: 27587722 Free PMC article.
-
RE: "INVITED COMMENTARY: BEWARE THE TEST-NEGATIVE DESIGN".Am J Epidemiol. 2017 Apr 1;185(7):613. doi: 10.1093/aje/kww227. Am J Epidemiol. 2017. PMID: 28338844 No abstract available.
References
-
- Barr IG, McCauley J, Cox N et al. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 Northern Hemisphere season. Vaccine. 2010;285:1156–1167. - PubMed
-
- Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;3117:2165–2168. - PubMed
-
- Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med. 1980;30310:549–552. - PubMed
-
- Skowronski D, Gilbert M, Tweed S et al. Effectiveness of vaccine against medical consultation due to laboratory-confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004–2005. Can Commun Dis Rep. 2005;3118:181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

