Measuring Ca2+-Dependent Modulation of Voltage-Gated Ca2+ Channels in HEK-293T Cells
- PMID: 27587775
- PMCID: PMC5162803
- DOI: 10.1101/pdb.prot087213
Measuring Ca2+-Dependent Modulation of Voltage-Gated Ca2+ Channels in HEK-293T Cells
Abstract
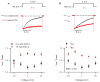
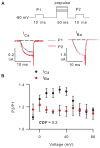
Voltage-gated Ca(2+) (Cav) channels regulate a variety of biological processes, such as muscle contraction, gene expression, and neurotransmitter release. Cav channels are subject to diverse forms of regulation, including those involving the Ca(2+) ions that permeate the pore. High voltage-activated Cav channels undergo Ca(2+)-dependent inactivation (CDI) and facilitation (CDF), which can regulate processes such as cardiac rhythm and synaptic plasticity. CDI and CDF differ slightly between Cav1 (L-type) and Cav2 (P/Q-, N-, and R-type) channels. Human embryonic kidney cells transformed with SV40 large T-antigen (HEK-293T) are advantageous for studying CDI and CDF of a particular type of Cav channel. HEK-293T cells do not express endogenous Cav channels, but Cav channels can be expressed exogenously at high levels in these cells by transient transfection. This protocol describes how to characterize and analyze Ca(2+)-dependent modulation of recombinant Cav channels in HEK-293T cells.
© 2016 Cold Spring Harbor Laboratory Press.
Figures
Similar articles
-
Ca2+-dependent modulation of voltage-gated Ca2+ channels.Biochim Biophys Acta. 2012 Aug;1820(8):1243-52. doi: 10.1016/j.bbagen.2011.12.012. Epub 2011 Dec 24. Biochim Biophys Acta. 2012. PMID: 22223119 Free PMC article. Review.
-
Large Ca²⁺-dependent facilitation of Ca(V)2.1 channels revealed by Ca²⁺ photo-uncaging.J Physiol. 2015 Jul 1;593(13):2753-78. doi: 10.1113/JP270091. J Physiol. 2015. PMID: 25809476 Free PMC article.
-
Whole-cell patch-clamp recording of recombinant voltage-sensitive Ca2+ channels heterologously expressed in HEK-293 cells.Cold Spring Harb Protoc. 2014 Apr 1;2014(4):396-401. doi: 10.1101/pdb.prot073213. Cold Spring Harb Protoc. 2014. PMID: 24692488
-
Decoding of synaptic voltage waveforms by specific classes of recombinant high-threshold Ca(2+) channels.J Physiol. 2003 Dec 1;553(Pt 2):473-88. doi: 10.1113/jphysiol.2003.051110. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500770 Free PMC article.
-
The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology.Endocr Rev. 2006 Oct;27(6):621-76. doi: 10.1210/er.2005-0888. Epub 2006 Jul 25. Endocr Rev. 2006. PMID: 16868246 Review.
Cited by
-
CaBP1 regulates Cav1 L-type Ca2+ channels and their coupling to neurite growth and gene transcription in mouse spiral ganglion neurons.Mol Cell Neurosci. 2018 Apr;88:342-352. doi: 10.1016/j.mcn.2018.03.005. Epub 2018 Mar 13. Mol Cell Neurosci. 2018. PMID: 29548764 Free PMC article.
-
Complex regulation of Cav2.2 N-type Ca2+ channels by Ca2+ and G-proteins.PLoS One. 2025 Feb 7;20(2):e0314839. doi: 10.1371/journal.pone.0314839. eCollection 2025. PLoS One. 2025. PMID: 39919080 Free PMC article.
-
Histamine via histamine H1 receptor enhances the muscarinic receptor-induced calcium response to acetylcholine in an enterochromaffin cell model.Clin Exp Pharmacol Physiol. 2022 Oct;49(10):1059-1071. doi: 10.1111/1440-1681.13682. Epub 2022 Jul 13. Clin Exp Pharmacol Physiol. 2022. PMID: 35652717 Free PMC article.
-
-254C>G SNP in the TRPC6 Gene Promoter Influences Its Expression via Interaction with the NF-κB Subunit RELA in Steroid-Resistant Nephrotic Syndrome Children.Int J Genomics. 2019 Jun 10;2019:2197837. doi: 10.1155/2019/2197837. eCollection 2019. Int J Genomics. 2019. PMID: 31281825 Free PMC article.
-
The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond.Int J Mol Sci. 2019 Jan 29;20(3):577. doi: 10.3390/ijms20030577. Int J Mol Sci. 2019. PMID: 30699993 Free PMC article. Review.
References
-
- DeMaria CD, Soong T, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
