Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A
- PMID: 27587878
- PMCID: PMC5073181
- DOI: 10.1182/blood-2016-04-713289
Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A
Abstract
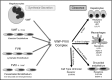
A normal hemostatic response to vascular injury requires both factor VIII (FVIII) and von Willebrand factor (VWF). In plasma, VWF and FVIII normally circulate as a noncovalent complex, and each has a critical function in the maintenance of hemostasis. Furthermore, the interaction between VWF and FVIII plays a crucial role in FVIII function, immunogenicity, and clearance, with VWF essentially serving as a chaperone for FVIII. Several novel recombinant FVIII (rFVIII) therapies for hemophilia A have been in clinical development, which aim to increase the half-life of FVIII (∼12 hours) and reduce dosing frequency by utilizing bioengineering techniques including PEGylation, Fc fusion, and single-chain design. However, these approaches have achieved only moderate increases in half-life of 1.5- to 2-fold compared with marketed FVIII products. Clearance of PEGylated rFVIII, rFVIIIFc, and rVIII-SingleChain is still regulated to a large extent by interaction with VWF. Therefore, the half-life of VWF (∼15 hours) appears to be the limiting factor that has confounded attempts to extend the half-life of rFVIII. A greater understanding of the interaction between FVIII and VWF is required to drive novel bioengineering strategies for products that either prolong the survival of VWF or limit VWF-mediated clearance of FVIII.
Figures


References
-
- Do H, Healey JF, Waller EK, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem. 1999;274(28):19587–19592. - PubMed
-
- Jacquemin M, Neyrinck A, Hermanns MI, et al. FVIII production by human lung microvascular endothelial cells. Blood. 2006;108(2):515–517. - PubMed
-
- Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004;11(4):272–277. - PubMed
-
- Terraube V, O’Donnell JS, Jenkins PV. Factor VIII and von Willebrand factor interaction: biological, clinical and therapeutic importance. Haemophilia. 2010;16(1):3–13. - PubMed
-
- Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood. 2004;104(5):1243–1252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

