Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model
- PMID: 27587968
- PMCID: PMC5007406
- DOI: 10.3348/kjr.2016.17.5.779
Therapeutic Effects of Microbubbles Added to Combined High-Intensity Focused Ultrasound and Chemotherapy in a Pancreatic Cancer Xenograft Model
Abstract
Objective: To investigate whether high-intensity focused ultrasound (HIFU) combined with microbubbles enhances the therapeutic effects of chemotherapy.
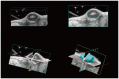
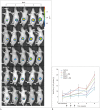
Materials and methods: A pancreatic cancer xenograft model was established using BALB/c nude mice and luciferase-expressing human pancreatic cancer cells. Mice were randomly assigned to five groups according to treatment: control (n = 10), gemcitabine alone (GEM; n = 12), HIFU with microbubbles (HIFU + MB, n = 11), combined HIFU and gemcitabine (HIGEM; n = 12), and HIGEM + MB (n = 13). After three weekly treatments, apoptosis rates were evaluated using the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay in two mice per group. Tumor volume and bioluminescence were monitored using high-resolution 3D ultrasound imaging and in vivo bioluminescence imaging for eight weeks in the remaining mice.
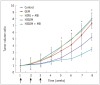
Results: The HIGEM + MB group showed significantly higher apoptosis rates than the other groups (p < 0.05) and exhibited the slowest tumor growth. From week 5, the tumor-volume-ratio relative to the baseline tumor volume was significantly lower in the HIGEM + MB group than in the control, GEM, and HIFU + MB groups (p < 0.05). Despite visible distinction, the HIGEM and HIGEM + MB groups showed no significant differences.
Conclusion: High-intensity focused ultrasound combined with microbubbles enhances the therapeutic effects of gemcitabine chemotherapy in a pancreatic cancer xenograft model.
Keywords: Animal study; Gemcitabine; High-intensity focused ultrasound; Microbubbles; Pancreatic cancer; Sonoporation.
Figures



Similar articles
-
Pulsed high-intensity focused ultrasound enhances apoptosis of pancreatic cancer xenograft with gemcitabine.Ultrasound Med Biol. 2013 Nov;39(11):1991-2000. doi: 10.1016/j.ultrasmedbio.2013.06.004. Epub 2013 Aug 22. Ultrasound Med Biol. 2013. PMID: 23972483
-
Antitumor effect of VEGFR2-targeted microbubble destruction with gemcitabine using an endoscopic ultrasound probe: In vivo mouse pancreatic ductal adenocarcinoma model.Hepatobiliary Pancreat Dis Int. 2020 Oct;19(5):478-485. doi: 10.1016/j.hbpd.2020.03.004. Epub 2020 Mar 20. Hepatobiliary Pancreat Dis Int. 2020. PMID: 32265136
-
A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer.J Control Release. 2016 Dec 10;243:172-181. doi: 10.1016/j.jconrel.2016.10.007. Epub 2016 Oct 12. J Control Release. 2016. PMID: 27744037 Clinical Trial.
-
Inoperable pancreatic adenocarcinoma rendered complete remission by high-intensity focused ultrasound concurrent with gemcitabine-capecitabine chemotherapy: case report and topic review.J Dig Dis. 2012 Jan;13(1):60-4. doi: 10.1111/j.1751-2980.2011.00546.x. J Dig Dis. 2012. PMID: 22188918 Review. No abstract available.
-
Microorganisms in chemotherapy for pancreatic cancer: An overview of current research and future directions.Int J Biol Sci. 2021 Jun 26;17(10):2666-2682. doi: 10.7150/ijbs.59117. eCollection 2021. Int J Biol Sci. 2021. PMID: 34326701 Free PMC article. Review.
Cited by
-
A portable high-intensity focused ultrasound system for the pancreas with 3D electronic steering: a preclinical study in a swine model.Ultrasonography. 2018 Oct;37(4):298-306. doi: 10.14366/usg.17048. Epub 2017 Oct 13. Ultrasonography. 2018. PMID: 29166762 Free PMC article.
-
Efficacy analysis of C5V chemotherapy combined with transcatheter subcutaneous radiofrequency ablation in the treatment of children with advanced (Stage III/IV) hepatoblastoma.Pak J Med Sci. 2022 Sep-Oct;38(7):1802-1807. doi: 10.12669/pjms.38.7.5899. Pak J Med Sci. 2022. PMID: 36246706 Free PMC article.
-
Combination of chemotherapy and focused ultrasound for the treatment of unresectable pancreatic cancer: a proof-of-concept study.Eur Radiol. 2023 Apr;33(4):2620-2628. doi: 10.1007/s00330-022-09271-8. Epub 2022 Dec 8. Eur Radiol. 2023. PMID: 36482217
-
High-intensity focused ultrasound therapy for pancreatic cancer.J Med Ultrason (2001). 2022 May 12. doi: 10.1007/s10396-022-01208-4. Online ahead of print. J Med Ultrason (2001). 2022. PMID: 35551555 Review.
-
Novel Therapeutic Method for Unresectable Pancreatic Cancer-The Impact of the Long-Term Research in Therapeutic Effect of High-Intensity Focused Ultrasound (HIFU) Therapy.Curr Oncol. 2021 Nov 20;28(6):4845-4861. doi: 10.3390/curroncol28060409. Curr Oncol. 2021. PMID: 34898585 Free PMC article.
References
-
- von Wichert G, Seufferlein T, Adler G. Palliative treatment of pancreatic cancer. J Dig Dis. 2008;9:1–7. - PubMed
-
- Cardenes HR, Chiorean EG, Dewitt J, Schmidt M, Loehrer P. Locally advanced pancreatic cancer: current therapeutic approach. Oncologist. 2006;11:612–623. - PubMed
-
- Maréchal R, Bachet JB, Mackey JR, Dalban C, Demetter P, Graham K, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012;143:664–674. - PubMed
-
- el-Kamar FG, Grossbard ML, Kozuch PS. Metastatic pancreatic cancer: emerging strategies in chemotherapy and palliative care. Oncologist. 2003;8:18–34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

