Failed reinnervation in aging skeletal muscle
- PMID: 27588166
- PMCID: PMC5007704
- DOI: 10.1186/s13395-016-0101-y
Failed reinnervation in aging skeletal muscle
Abstract
Background: Skeletal muscle displays a marked accumulation of denervated myofibers at advanced age, which coincides with an acceleration of muscle atrophy.
Methods: In this study, we evaluated the hypothesis that the accumulation of denervated myofibers in advanced age is due to failed reinnervation by examining muscle from young adult (YA) and very old (VO) rats and from a murine model of sporadic denervation secondary to neurotrypsin over-expression (Sarco mouse).
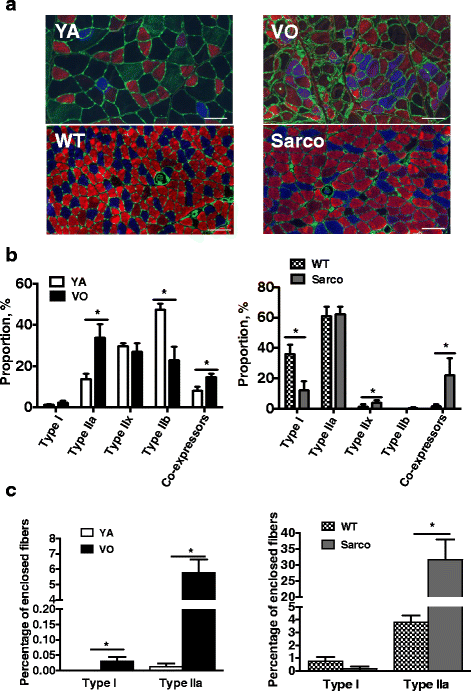
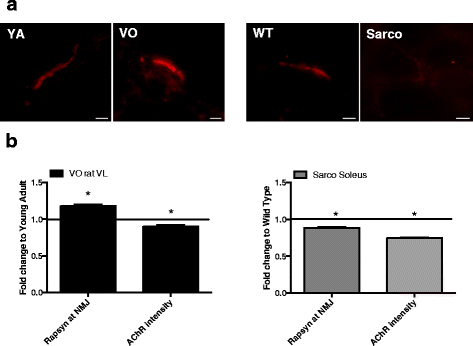
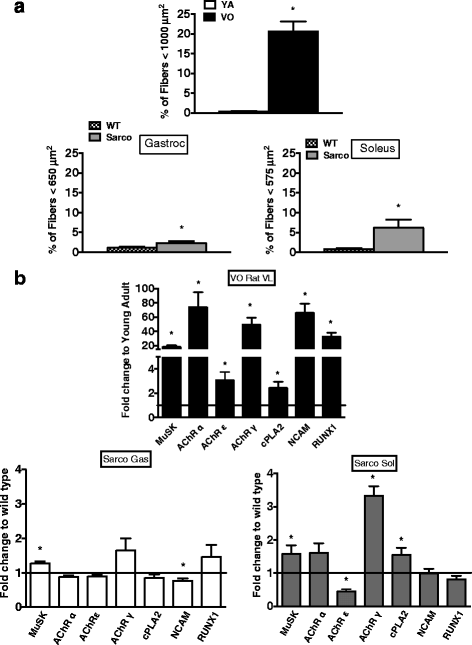
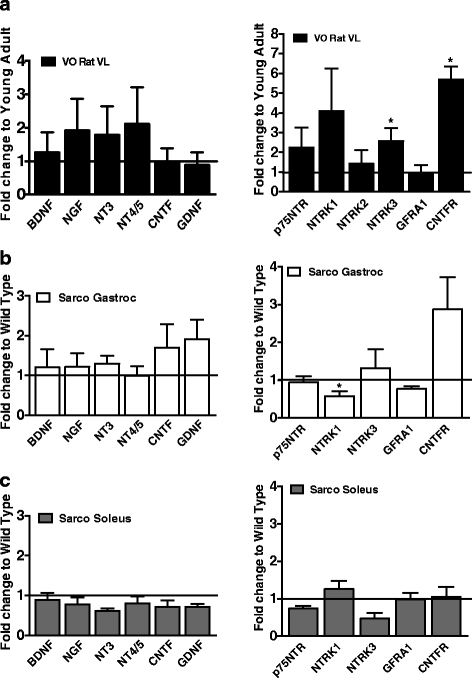
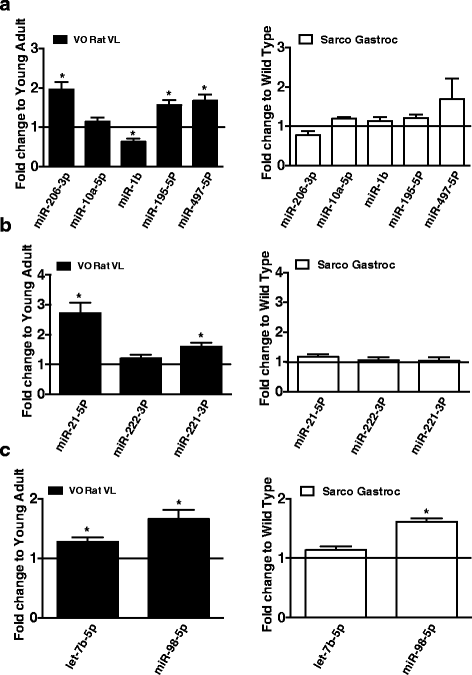
Results: Both aging rat muscle and Sarco mouse muscle exhibited marked fiber-type grouping, consistent with repeating cycles of denervation and reinnervation, yet in VO muscle, rapsyn at the endplate increased and was associated with only a 10 % decline in acetylcholine receptor (AChR) intensity, whereas in Sarco mice, there was a decline in rapsyn and a 25 % decrease in AChR intensity. Transcripts of muscle-specific kinase (21-fold), acetylcholine receptor subunits α (68-fold), ε (threefold) and γ (47-fold), neural cell adhesion molecule (66-fold), and runt-related transcription factor 1 (33-fold) were upregulated in VO muscle of the rat, consistent with the marked persistent denervation evidenced by a large proportion of very small fibers (>20 %). In the Sarco mice, there were much smaller increases in denervation transcripts (0-3.5-fold) and accumulation of very small fibers (2-6 %) compared to the VO rat, suggesting a reduced capacity for reinnervation in aging muscle. Despite the marked persistent denervation in the VO rat muscle, transcripts of neurotrophins involved in promoting axonal sprouting following denervation exhibited no increase, and several miRNAs predicted to suppress neurotrophins were elevated in VO rat.
Conclusions: Our results support the hypothesis that the accumulation of denervated fibers with aging is due to failed reinnervation and that this may be affected by a limited neurotrophin response that mediates axonal sprouting following denervation.
Keywords: Aging; Denervation; MicroRNA; Muscle atrophy; Neurotrophins; Reinnervation; Sarcopenia; Skeletal muscle.
Figures
References
-
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci. 1995;50:91–95. - PubMed
-
- Rowan SL, Purves-Smith FM, Solbak NM, Hepple RT. Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp Gerontol. 2011;46:660–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

