Prospective studies of infectious mononucleosis in university students
- PMID: 27588199
- PMCID: PMC5007628
- DOI: 10.1038/cti.2016.48
Prospective studies of infectious mononucleosis in university students
Abstract
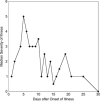
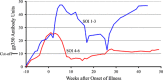
We performed an intensive prospective study designed to obtain as much data as possible on the incubation and early illness periods of primary Epstein-Barr virus (EBV) infection. Undergraduate students who lacked EBV antibody and oral EBV DNA (EBV-naive) were seen every 2 weeks during their freshman year. Clinical and behavioral data, oral washes and venous blood were obtained. EBV antibodies were quantified by enzyme immunoassay and viral loads by PCR. During a median 8 months of observation, 14/85 subjects experienced primary EBV infections (24 cases/100 person-years). The only significant risk factor for acquisition of EBV infection was deep kissing (P=0.02). Eleven subjects had infectious mononucleosis with a median duration of 21 days. Two subjects were hospitalized. Infections were initially identified in 12 subjects by finding EBV DNA in oral cells before onset of symptoms and in 2 subjects by symptom reporting. EBV DNA and viral capsid antigen (VCA) IgM and gp350 IgG antibodies were present in the blood before onset of illness. To provide a more robust evaluation of primary EBV infection in undergraduate university students, we combined data on risk factors and antibody responses from this and an earlier study that used the exact same clinical and laboratory methods. The observation that the only significant risk factor for acquisition of EBV infection was deep kissing was confirmed. Most importantly, higher amounts of gp350 antibody correlated significantly with a lower severity of infectious mononucleosis (P<0.0001), which strengthens the rationale for a gp350-based prophylactic EBV vaccine.
Figures


References
-
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet 1964; 1: 702–703. - PubMed
-
- de-The G, Day NE, Geser A, Lavoue MF, Ho JH, Simons MJ et al. Sero-epidemiology of the Epstein–Barr virus: preliminary analysis of an international study—a review. IARC Sci Publ 1975; 11(Pt 2): 3–16. - PubMed
-
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein–Barr virus vaccine in healthy young adults. J Infect Dis 2007; 196: 1749–1753. - PubMed
-
- Crawford DH, Macsween KF, Higgins CD, Thomas R, McAulay K, Williams H et al. A cohort study among university students: identification of risk factors for Epstein–Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis 2006; 43: 276–282. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
