Excessive Sensory Stimulation during Development Alters Neural Plasticity and Vulnerability to Cocaine in Mice
- PMID: 27588306
- PMCID: PMC4994069
- DOI: 10.1523/ENEURO.0199-16.2016
Excessive Sensory Stimulation during Development Alters Neural Plasticity and Vulnerability to Cocaine in Mice
Abstract
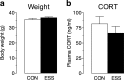
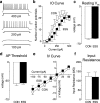
Early life experiences affect the formation of neuronal networks, which can have a profound impact on brain function and behavior later in life. Previous work has shown that mice exposed to excessive sensory stimulation during development are hyperactive and novelty seeking, and display impaired cognition compared with controls. In this study, we addressed the issue of whether excessive sensory stimulation during development could alter behaviors related to addiction and underlying circuitry in CD-1 mice. We found that the reinforcing properties of cocaine were significantly enhanced in mice exposed to excessive sensory stimulation. Moreover, although these mice displayed hyperactivity that became more pronounced over time, they showed impaired persistence of cocaine-induced locomotor sensitization. These behavioral effects were associated with alterations in glutamatergic transmission in the nucleus accumbens and amygdala. Together, these findings suggest that excessive sensory stimulation in early life significantly alters drug reward and the neural circuits that regulate addiction and attention deficit hyperactivity. These observations highlight the consequences of early life experiences and may have important implications for children growing up in today's complex technological environment.
Keywords: amygdala; drug addiction; environment; mice; nucleus accumbens; sensory stimulation.
Figures



Similar articles
-
Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine.Nat Neurosci. 2001 Dec;4(12):1217-23. doi: 10.1038/nn757. Nat Neurosci. 2001. PMID: 11694884
-
Cocaine exposure enhances excitatory synaptic drive to cholinergic neurons in the laterodorsal tegmental nucleus.Eur J Neurosci. 2013 Oct;38(7):3027-35. doi: 10.1111/ejn.12296. Epub 2013 Jul 3. Eur J Neurosci. 2013. PMID: 23822660
-
Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens.J Neurosci. 2007 Jul 25;27(30):7921-8. doi: 10.1523/JNEUROSCI.1859-07.2007. J Neurosci. 2007. PMID: 17652583 Free PMC article.
-
Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction.Ann N Y Acad Sci. 2010 Feb;1187:35-75. doi: 10.1111/j.1749-6632.2009.05144.x. Ann N Y Acad Sci. 2010. PMID: 20201846 Free PMC article. Review.
-
Impact of juvenile chronic stress on adult cortico-accumbal function: Implications for cognition and addiction.Prog Neuropsychopharmacol Biol Psychiatry. 2017 Oct 3;79(Pt B):136-154. doi: 10.1016/j.pnpbp.2017.06.015. Epub 2017 Jun 19. Prog Neuropsychopharmacol Biol Psychiatry. 2017. PMID: 28642080 Free PMC article. Review.
Cited by
-
Sex-dependent effects of early life sensory overstimulation on later life behavioral function in rats.Sci Rep. 2024 Nov 12;14(1):27650. doi: 10.1038/s41598-024-78928-9. Sci Rep. 2024. PMID: 39532944 Free PMC article.
-
Sensory seeking and its influence on sustained attention performance in adult males with Autism Spectrum Condition.Sci Rep. 2025 Feb 3;15(1):4047. doi: 10.1038/s41598-025-88733-7. Sci Rep. 2025. PMID: 39901051 Free PMC article.
-
How early media exposure may affect cognitive function: A review of results from observations in humans and experiments in mice.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):9851-9858. doi: 10.1073/pnas.1711548115. Proc Natl Acad Sci U S A. 2018. PMID: 30275319 Free PMC article. Review.
References
-
- Aggleton JP (2000) The amygdala: a functional analysis. New York: Oxford UP.[TQ1][TQ2]
-
- Anand A, Shekhar A (2003) Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci 985:370–388. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
