DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations
- PMID: 27588403
- PMCID: PMC5325442
- DOI: 10.18632/oncotarget.11733
DMBA induced mouse mammary tumors display high incidence of activating Pik3caH1047 and loss of function Pten mutations
Abstract
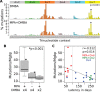
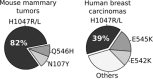
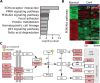
Controversy always existed on the utility of chemically induced mouse mammary carcinogenesis models as valid equivalents for the study of human breast cancer. Here, we performed whole exome and RNA sequencing on long latency mammary tumors (218 ± 27 days) induced by the carcinogen 7,12-Dimethylbenzathracene (DMBA) and short latency tumors (65 ± 11 days) induced by the progestin Medroxyprogesterone Acetate (MPA) plus DMBA in CD2F1 mice. Long latency tumors displayed a high frequency of Pi3kca and/or Pten mutations detected in 11 of 13 (85%) long latency cases (14/22, 64% overall). Eighty-two percent (9/11) of tumors carried the Pik3ca H1047L/R hot-spot mutation, as frequently found in human breast cancer. These tumors were luminal-like and mostly ER/PR+, as in humans. Transcriptome profiling indicated a significant activation of the PI3K-Akt pathway (p=3.82e-6). On the other hand MPA+DMBA induced short latency tumors displayed mutations in cancer drivers not commonly found mutated in human breast cancer (e.g. Hras and Apc). These tumors were mostly basal-like and MPA exposure led to Rankl overexpression (60 fold induction) and immunosuppressive gene expression signatures. In summary, long latency DMBA induced mouse mammary tumors reproduce the molecular profile of human luminal breast carcinomas representing an excellent preclinical model for the testing of PIK3CA/Akt/mTOR pathway inhibitory therapies and a good platform for the developing of additional preclinical tools such as syngeneic transplants in immunocompetent hosts.
Keywords: DMBA; MPA; Pik3ca; Pten; mammary tumors.
Conflict of interest statement
The authors disclose no conflicts of interest.
Figures




References
-
- Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112. - PubMed
-
- Lanari C, Molinolo AA, Pasqualini CD. Induction of mammary adenocarcinomas by medroxyprogesterone acetate in BALBc female mice. Cancer letters. 1986;33:215–223. - PubMed
-
- Aldaz CM, Liao QY, LaBate M, Johnston DA. Medroxyprogesterone acetate accelerates the development and increases the incidence of mouse mammary tumors induced by dimethylbenzanthracene. Carcinogenesis. 1996;17:2069–2072. - PubMed
-
- Sukumar S, Notario V, Martin-Zanca D, Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1982;306:658–61. - PubMed
-
- Zarbl H, Sukumar S, Arthur AV, Martin-Zanca D, Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. Nature. 1985;315:382–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

