Long-Term Efficacy, Safety, and Pharmacokinetics of Drisapersen in Duchenne Muscular Dystrophy: Results from an Open-Label Extension Study
- PMID: 27588424
- PMCID: PMC5010191
- DOI: 10.1371/journal.pone.0161955
Long-Term Efficacy, Safety, and Pharmacokinetics of Drisapersen in Duchenne Muscular Dystrophy: Results from an Open-Label Extension Study
Abstract
Background: Drisapersen induces exon 51 skipping during dystrophin pre-mRNA splicing and allows synthesis of partially functional dystrophin in Duchenne muscular dystrophy (DMD) patients with amenable mutations.
Methods: This 188-week open-label extension of the dose-escalation study assessed the long-term efficacy, safety, and pharmacokinetics of drisapersen (PRO051/GSK2402968), 6 mg/kg subcutaneously, in 12 DMD subjects. Dosing was once weekly for 72 weeks. All subjects had a planned treatment interruption (weeks 73-80), followed by intermittent dosing (weeks 81-188).
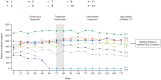
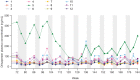
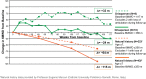
Results: Subjects received a median (range) total dose of 5.93 (5.10 to 6.02) mg/kg drisapersen. After 177 weeks (last efficacy assessment), median (mean [SD]) six-minute walk distance (6MWD) improved by 8 (-24.5 [161]) meters for the 10 subjects able to complete the 6MWD at baseline (mean age [SD]: 9.5 [1.9] years). These statistics include 2 subjects unable to complete the test at later visits and who scored "zero". When only the 8 ambulant subjects at week 177 were taken into account, a median (mean [SD]) increase of 64 (33 [121]) meters in 6MWD was observed. Of 7 subjects walking ≥330 m at extension baseline, 5 walked farther at week 177. Of 3 subjects walking <330 m, 2 lost ambulation, while 1 declined overall but walked farther at some visits. Over the 188 weeks, the most common adverse events were injection-site reactions, raised urinary α1-microglobulin and proteinuria. Dystrophin expression was detected in all muscle biopsies obtained at week 68 or 72.
Conclusion: Drisapersen was generally well tolerated over 188 weeks. Possible renal effects, thrombocytopenia and injection-site reactions warrant continued monitoring. Improvements in the 6MWD at 12 weeks were sustained after 3.4 years of dosing for most patients. For a small, uncontrolled study, the outcomes are encouraging, as natural history studies would anticipate a decline of over 100 meters over a 3-year period in a comparable cohort.
Trial registration: ClinicalTrials.gov NCT01910649.
Conflict of interest statement
Competing Interests: Nathalie M. Goemans has received funding for trials from Prosensa Therapeutics BV limited to the study costs. Rosamund J. Wilson was an employee of Spica Consultants Ltd, Marlborough, UK, and had a consultancy contract with Prosensa Therapeutics BV. Judith C. van Deutekom, Sjef J. de Kimpe, Afrodite Lourbakos and Giles V. Campion were employees (which includes contribution to patent [applications] and participation in stock-option plans) of Prosensa Therapeutics BV. Judith C. van Deutekom, Sjef J. de Kimpe, Afrodite Lourbakos and Giles V. Campion are employees (which includes participation in stock-option plans) of BioMarin Pharamaceutical Inc. None of the contributing authors listed on this paper were affiliated with GlaxoSmithKline at the time of the study. Már Tulinius, Marleen van den Hauwe, Anna-Karin Kroksmark, and Gunnar Buyse have declared that no competing interests exist. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Emery AE. Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul.Disord. 1991;1:19–29. - PubMed
-
- Henricson E, Abresch R, Han JJ, Nicorici A, Goude Keller E, de Bie E et al. The 6-minute walk test and person-reported outcomes in boys with duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically-meaningful changes over one year. PLoS.Curr. 2013;5: - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

