The underlying inflammatory chronic disease influences infliximab pharmacokinetics
- PMID: 27589009
- PMCID: PMC5058621
- DOI: 10.1080/19420862.2016.1216741
The underlying inflammatory chronic disease influences infliximab pharmacokinetics
Abstract
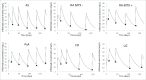
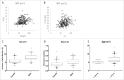
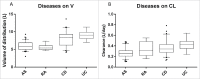
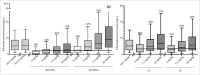
Infliximab is an anti-tumor necrosis factor monoclonal antibody approved in chronic inflammatory diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), Crohn's disease (CD) and ulcerative colitis (UC). Infliximab pharmacokinetics is variable between patients, but influence of the underlying disease was never assessed. This study aimed at assessing this influence using a cohort of patients monitored in a single center and with the same assay. Infliximab trough concentrations were determined on samples collected between weeks 0 and 22 after treatment initiation in 218 patients treated for RA, PsA, AS, CD or UC. Infliximab pharmacokinetics was analyzed by a one-compartment population model with first-order elimination rate constant. In AS patients, volume of distribution (V) and elimination clearance (CL) were 5.4 L and 0.24 L/day, respectively. In CD and UC patients, V was 49% and 52% higher than in AS, respectively, and CL was 47% and 60% higher than in AS, respectively. In RA patients, CL was 49% higher than in AS patients. Simulations showed that without methotrexate, a 3 mg/kg dosing regimen would lead only 16% of RA patients to reach the target concentration (2.5 mg/L) at week 22, whereas target concentrations would be reached in approximately half of RA patients cotreated with methotrexate, as well as half of CD (3.5 mg/L) and UC (3.7 mg/L) patients. The suboptimality of approved dosing regimens supports the development of dosing optimization based on concentration measurements.
Keywords: Ankylosing spondylitis; Crohn's disease; inflammatory bowel disease; infliximab; monoclonal antibodies; pharmacokinetics; psoriatic arthritis; rheumatoid arthritis; therapeutic drug monitoring; ulcerative colitis.
Figures


References
-
- St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, Keystone EC. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2002; 46:1451-9; PMID:12115174; http://dx.doi.org/10.1002/art.10302 - DOI - PubMed
-
- Krzysiek R, Breban M, Ravaud P, Prejean MV, Wijdenes J, Roy C, Henry YD, Barbey C, Trappe G, Dougados M, et al.. Circulating concentration of infliximab and response to treatment in ankylosing spondylitis: results from a randomized control study. Arthritis Rheum 2009; 61:569-76; PMID:19405015; http://dx.doi.org/10.1002/art.24275 - DOI - PubMed
-
- Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, Claes K, Coopman T, Van Schuerbeek N, Van Assche G, et al.. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology 2009; 137:1628-40; PMID:19664627; http://dx.doi.org/10.1053/j.gastro.2009.07.062 - DOI - PubMed
-
- Ternant D, Ducourau E, Perdriger A, Corondan A, Le Goff B, Devauchelle-Pensec V, Solau-Gervais E, Watier H, Goupille P, Paintaud G, et al.. Relationship between inflammation and infliximab pharmacokinetics in rheumatoid arthritis. Br J Clin Pharmacol 2014; 78:118-28; PMID:24354889; http://dx.doi.org/10.1111/bcp.12313 - DOI - PMC - PubMed
-
- Xu Z, Seitz K, Fasanmade A, Ford J, Williamson P, Xu W, Davis HM, Zhou H. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol 2008; 48:681-95; PMID:18401017; http://dx.doi.org/10.1177/0091270008316886 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
