Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with Type 2 diabetes: the DUAL IV trial
- PMID: 27589252
- PMCID: PMC5811787
- DOI: 10.1111/dme.13256
Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with Type 2 diabetes: the DUAL IV trial
Abstract
Aim: To investigate the safety and efficacy of insulin degludec/liraglutide (IDegLira), a novel combination product, as add-on therapy for people with Type 2 diabetes uncontrolled on sulphonylurea therapy.
Methods: In this 26-week, double-blind trial, adults with Type 2 diabetes [HbA1c 53-75 mmol/mol (7.0-9.0%)] were randomized to IDegLira (n = 289) or placebo (n = 146) as add-on to pre-trial sulphonylurea ± metformin, titrating to a fasting glycaemic target of 4.0-6.0 mmol/l. Treatment initiation was at 10 dose steps, and maximum dose was 50 dose steps (50 units insulin degludec/1.8 mg liraglutide).
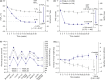
Results: The mean HbA1c decreased from 63 mmol/mol (7.9%) to 46 mmol/mol (6.4%) with IDegLira and to 57 mmol/mol (7.4%) with placebo [estimated treatment difference -11 mmol/mol (95% CI -13; -10) or -1.02% (95% CI -1.18; -0.87); P < 0.001]. The HbA1c target of 53 mmol/mol (<7%) was achieved by 79.2% of participants in the IDegLira group vs 28.8% in the placebo group [estimated odds ratio 11.95 (95% CI 7.22; 19.77); P < 0.001]. Mean weight change was +0.5 kg with IDegLira vs -1.0 kg with placebo [estimated treatment difference 1.48 kg (95% CI 0.90; 2.06); P < 0.001]. Confirmed hypoglycaemia occurred in 41.7 and 17.1% of IDegLira- and placebo-treated participants, respectively, with rates of 3.5 vs 1.4 events/patient-years of exposure [estimated rate ratio 3.74 (95% CI 2.28; 6.13); P < 0.001]. IDegLira was generally well tolerated. The rates of serious adverse events were 20.3 and 8.0 per 100 patient-years of exposure with IDegLira and placebo, respectively, without obvious patterns in the type of events.
Conclusions: IDegLira can be used in people uncontrolled with sulphonylurea ± metformin to improve efficacy with a safety profile in line with previous DUAL trials.
© 2016 Diabetes UK.
Figures

References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M et al Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012; 55: 1577–1596. - PubMed
-
- Holman RR. Assessing the potential for alpha‐glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract 1998; 40(Suppl.): S21–S25. - PubMed
-
- Rodbard HW, Jellinger PS, Davidson JA, Einhorn D, Garber AJ, Grunberger G et al Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15: 540–559. - PubMed
-
- Spollett GR. Insulin initiation in type 2 diabetes: what are the treatment regimen options and how can we best help patients feel empowered? J Am Acad Nurse Pract 2012; 24(Suppl. 1): 249–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

