Development of an excitatory kisspeptin projection to the oxytocin system in late pregnancy
- PMID: 27589336
- PMCID: PMC5285723
- DOI: 10.1113/JP273051
Development of an excitatory kisspeptin projection to the oxytocin system in late pregnancy
Abstract
Key points: Oxytocin release from the posterior pituitary gland stimulates uterine contraction during birth but the central mechanisms that activate oxytocin neurones for birth are not well characterized. We found that that kisspeptin fibre density around oxytocin neurones increases in late-pregnant rats. These kisspeptin fibres originated from hypothalamic periventricular nucleus neurones that upregulated kisspeptin expression in late pregnancy. Oxytocin neurones were excited by central kisspeptin administration in late-pregnant rats but not in non-pregnant rats or early- to mid-pregnant rats. Our results reveal the emergence of a new excitatory kisspeptin projection to the oxytocin system in late pregnancy that might contribute to oxytocin neurone activation for birth.
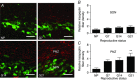
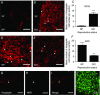
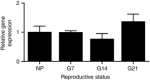
Abstract: The hormone oxytocin promotes uterine contraction during parturition. Oxytocin is synthesized by magnocellular neurones in the hypothalamic supraoptic and paraventricular nuclei and is released into the circulation from the posterior pituitary gland in response to action potential firing. Systemic kisspeptin administration increases oxytocin neurone activity to elevate plasma oxytocin levels. Here, immunohistochemistry revealed that rats on the expected day of parturition (day 21 of gestation) had a higher density of kisspeptin-positive fibres in the perinuclear zone surrounding the supraoptic nucleus (which provides dense glutamatergic and GABAergic innervation to the supraoptic nucleus) than was evident in non-pregnant rats. Retrograde tracing showed the kisspeptin projections to the perinuclear zone originated from the hypothalamic periventricular nucleus. Quantitative RT-PCR showed that kisspeptin receptor mRNA, Kiss1R mRNA, was expressed in the perinuclear zone-supraoptic nucleus and that the relative Kiss1R mRNA expression does not change over the course of pregnancy. Finally, intracerebroventricular administration of kisspeptin increased the firing rate of oxytocin neurones in anaesthetized late-pregnant rats (days 18-21 of gestation) but not in non-pregnant rats, or in early- or mid-pregnant rats. Taken together, these results suggest that kisspeptin expression is upregulated in the periventricular nucleus projection to the perinuclear zone of the supraoptic nucleus towards the end of pregnancy. Hence, this input might activate oxytocin neurones during parturition.
Keywords: kisspeptin; oxytocin; pregnancy; supraoptic nucleus; vasopressin.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures




Comment in
-
Kisspeptin: a new peptidergic system regulating oxytocin neurons and their reproductive plasticity in the hypothalamo-neurohypophysial system.J Physiol. 2017 Feb 1;595(3):611-612. doi: 10.1113/JP273364. J Physiol. 2017. PMID: 28145008 Free PMC article. No abstract available.
References
-
- Antonijevic IA, Douglas AJ, Dye S, Bicknell RJ, Leng G & Russell JA (1995). Oxytocin antagonists delay the initiation of parturition and prolong its active phase in rats. J Endocrinol 145, 97–103. - PubMed
-
- Armstrong WE & Stern JE (1997). Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol 78, 2427–2437. - PubMed
-
- Augustine RA, Bouwer GT, Seymour AJ, Grattan DR & Brown CH (2016). Reproductive regulation of gene expression in the hypothalamic supraoptic and paraventricular nuclei. J Neuroendocrinol 28, DOI: 10.1111/jne.12350. - PubMed
-
- Bianco SD & Kaiser UB (2013). Molecular biology of the kisspeptin receptor: signaling, function, and mutations. Adv Exp Med Biol 784, 133–158. - PubMed
-
- Boudaba C, Di S & Tasker JG (2003). Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol 15, 803–810. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

