Halting of Caspase Activity Protects Tau from MC1-Conformational Change and Aggregation
- PMID: 27589517
- PMCID: PMC5088409
- DOI: 10.3233/JAD-150960
Halting of Caspase Activity Protects Tau from MC1-Conformational Change and Aggregation
Abstract
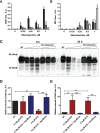
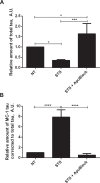
Intracellular neurofibrillary tangles (NFTs) are the hallmark of Alzheimer's disease and other tauopathies in which tau, a microtubule-associated protein, loses its ability to stabilize microtubules. Several post-translational modifications including phosphorylation and truncation increase tau's propensity to aggregate thus forming NFTs; however, the mechanisms underlying tau conformational change and aggregation still remain to be defined. Caspase activation and subsequent proteolytic cleavage of tau is thought to be a potential trigger of this disease-related pathological conformation. The aim of this work was to investigate the link between caspase activation and a disease-related conformational change of tau in a neuroblastoma cell-based model of spontaneous tau aggregation. We demonstrated that caspase induction initiates proteolytic cleavage of tau and generation of conformationally altered and aggregated tau recognized by the MC1 conformational antibody. Most importantly, these events were shown to be attenuated with caspase inhibitors. This implies that therapeutics aimed at inhibiting caspase-mediated tau cleavage may prove beneficial in slowing cleavage and aggregation, thus potentially halting tau pathology and disease progression.
Keywords: Alzheimer’s disease; caspases; neurodegenerative diseases; protein aggregation; tau proteins; tauopathies.
Figures

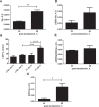

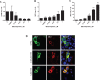
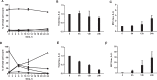
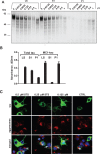
References
-
- Selkoe DJ (1986) Altered structural proteins in plaques and tangles: What do they tell us about the biology of Alzheimer’s disease? Neurobiol Aging 7, 4225–4232. - PubMed
-
- Yagishita S, Itoh Y, Nan W, Amano N (1981) Reappraisal of the fine structure of Alzheimer’s neurofibrillary tangles. Acta Neuropathol 54, 239–246. - PubMed
-
- Ihara Y, Nukina N, Miura R, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem 99, 1807–1810. - PubMed
-
- Cleveland DW, Hwo SY, Kirschner MW (1977) Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol 116, 227–247. - PubMed
-
- Avila J, Lucas JJ, Perez M, Hernandez F (2004) Role of tau protein in both physiological and pathological conditions. Physiol Rev 84, 361–384. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

