Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus
- PMID: 27589726
- PMCID: PMC5037708
- DOI: 10.3390/ijms17091429
Impact of Phosphate, Potassium, Yeast Extract, and Trace Metals on Chitosan and Metabolite Production by Mucor indicus
Abstract
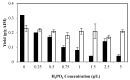
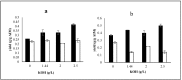
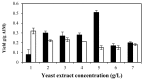
In this study the effects of phosphate, potassium, yeast extract, and trace metals on the growth of Mucor indicus and chitosan, chitin, and metabolite production by the fungus were investigated. Maximum yield of chitosan (0.32 g/g cell wall) was obtained in a phosphate-free medium. Reversely, cell growth and ethanol formation by the fungus were positively affected in the presence of phosphate. In a phosphate-free medium, the highest chitosan content (0.42 g/g cell wall) and cell growth (0.66 g/g sugar) were obtained at 2.5 g/L of KOH. Potassium concentration had no significant effect on ethanol and glycerol yields. The presence of trace metals significantly increased the chitosan yield at an optimal phosphate and potassium concentration (0.50 g/g cell wall). By contrast, production of ethanol by the fungus was negatively affected (0.33 g/g sugars). A remarkable increase in chitin and decrease in chitosan were observed in the absence of yeast extract and concentrations lower than 2 g/L. The maximum chitosan yield of 51% cell wall was obtained at 5 g/L of yeast extract when the medium contained no phosphate, 2.5 g/L KOH, and 1 mL/L trace metal solution.
Keywords: Mucor indicus; chitosan; ethanol; phosphate; potassium; trace metals; yeast extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Abtahi Z. Master’s Thesis. University College of Borås; Borås, Sweden: 2008. Ethanol and Glucose Tolerance of M. indicus in Aerobic and Anaerobic Conditions.
-
- Zamani A. Ph.D. Thesis. Chalmers University of Technology; Göteborg, Sweden: 2010. Superabsorbent Polymers from the Cell Wall of Zygomycetes Fungi.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

