Lipid Droplets: A Key Cellular Organelle Associated with Cancer Cell Survival under Normoxia and Hypoxia
- PMID: 27589734
- PMCID: PMC5037709
- DOI: 10.3390/ijms17091430
Lipid Droplets: A Key Cellular Organelle Associated with Cancer Cell Survival under Normoxia and Hypoxia
Abstract
The Warburg effect describes the phenomenon by which cancer cells obtain energy from glycolysis even under normoxic (O₂-sufficient) conditions. Tumor tissues are generally exposed to hypoxia owing to inefficient and aberrant vasculature. Cancer cells have multiple molecular mechanisms to adapt to such stress conditions by reprogramming the cellular metabolism. Hypoxia-inducible factors are major transcription factors induced in cancer cells in response to hypoxia that contribute to the metabolic changes. In addition, cancer cells within hypoxic tumor areas have reduced access to serum components such as nutrients and lipids. However, the effect of such serum factor deprivation on cancer cell biology in the context of tumor hypoxia is not fully understood. Cancer cells are lipid-rich under normoxia and hypoxia, leading to the increased generation of a cellular organelle, the lipid droplet (LD). In recent years, the LD-mediated stress response mechanisms of cancer cells have been revealed. This review focuses on the production and functions of LDs in various types of cancer cells in relation to the associated cellular environment factors including tissue oxygenation status and metabolic mechanisms. This information will contribute to the current understanding of how cancer cells adapt to diverse tumor environments to promote their survival.
Keywords: cancer; hypoxia; lipid droplets; normoxia; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
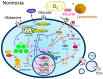
 : LCFAs,
: LCFAs,  : cholesterol,
: cholesterol,  : transporter,
: transporter,  : receptor.
: receptor.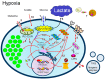
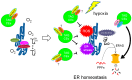

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

