Effects of Female Sex Hormones on Susceptibility to HSV-2 in Vaginal Cells Grown in Air-Liquid Interface
- PMID: 27589787
- PMCID: PMC5035955
- DOI: 10.3390/v8090241
Effects of Female Sex Hormones on Susceptibility to HSV-2 in Vaginal Cells Grown in Air-Liquid Interface
Abstract
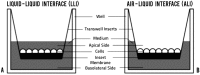
The lower female reproductive tract (FRT) is comprised of the cervix and vagina, surfaces that are continuously exposed to a variety of commensal and pathogenic organisms. Sexually transmitted viruses, such as herpes simplex virus type 2 (HSV-2), have to traverse the mucosal epithelial lining of the FRT to establish infection. The majority of current culture systems that model the host-pathogen interactions in the mucosal epithelium have limitations in simulating physiological conditions as they employ a liquid-liquid interface (LLI), in which both apical and basolateral surfaces are submerged in growth medium. We designed the current study to simulate in vivo conditions by growing an immortalized vaginal epithelial cell line (Vk2/E6E7) in culture with an air-liquid interface (ALI) and examined the effects of female sex hormones on their growth, differentiation, and susceptibility to HSV-2 under these conditions, in comparison to LLI cultures. ALI conditions induced Vk2/E6E7 cells to grow into multi-layered cultures compared to the monolayers present in LLI conditions. Vk2 cells in ALI showed higher production of cytokeratin in the presence of estradiol (E2), compared to cells grown in progesterone (P4). Cells grown under ALI conditions were exposed to HSV-2-green fluorescent protein (GFP) and the highest infection and replication was observed in the presence of P4. Altogether, this study suggests that ALI cultures more closely simulate the in vivo conditions of the FRT compared to the conventional LLI cultures. Furthermore, under these conditions P4 was found to confer higher susceptibility to HSV-2 infection in vaginal cells. The vaginal ALI culture system offers a better alternative to study host-pathogen interactions.
Keywords: HSV-2; Vk2/E6E7 cells; air-liquid interface; estradiol; female genital tract; female sex hormones; herpes simplex virus type 2; medroxyprogesterone acetate; progesterone; vaginal cells.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
Ex vivo 2D and 3D HSV-2 infection model using human normal vaginal epithelial cells.Oncotarget. 2017 Feb 28;8(9):15267-15282. doi: 10.18632/oncotarget.14840. Oncotarget. 2017. PMID: 28146426 Free PMC article.
-
Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection.Biol Reprod. 2007 Dec;77(6):1049-59. doi: 10.1095/biolreprod.107.063933. Epub 2007 Sep 19. Biol Reprod. 2007. PMID: 17881767
-
Differential responses of murine vaginal and uterine epithelial cells prior to and following herpes simplex virus type 2 (HSV-2) infection.Am J Reprod Immunol. 2007 May;57(5):367-77. doi: 10.1111/j.1600-0897.2007.00482.x. Am J Reprod Immunol. 2007. PMID: 17430501
-
Primary human epithelial cell culture system for studying interactions between female upper genital tract and sexually transmitted viruses, HSV-2 and HIV-1.Methods. 2011 Oct;55(2):114-21. doi: 10.1016/j.ymeth.2011.09.022. Epub 2011 Oct 4. Methods. 2011. PMID: 21996033 Review.
-
Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications.Hum Reprod Update. 2015 May-Jun;21(3):353-77. doi: 10.1093/humupd/dmu065. Epub 2014 Dec 29. Hum Reprod Update. 2015. PMID: 25547201 Review.
Cited by
-
Three-dimensional models of the cervicovaginal epithelia to study host-microbiome interactions and sexually transmitted infections.Pathog Dis. 2022 Aug 27;80(1):ftac026. doi: 10.1093/femspd/ftac026. Pathog Dis. 2022. PMID: 35927516 Free PMC article.
-
Conditional Cell Reprogramming and Air-Liquid Interface Modeling Life Cycle of Oncogenic Viruses (HPV and EBV) in Epithelial Cells and Virus-Associated Human Carcinomas.Viruses. 2023 Jun 17;15(6):1388. doi: 10.3390/v15061388. Viruses. 2023. PMID: 37376685 Free PMC article. Review.
-
LAMP3/CD63 Expression in Early and Late Endosomes in Human Vaginal Epithelial Cells Is Associated with Enhancement of HSV-2 Infection.J Virol. 2022 Dec 14;96(23):e0155322. doi: 10.1128/jvi.01553-22. Epub 2022 Nov 9. J Virol. 2022. PMID: 36350153 Free PMC article.
-
Recent advances in organoid development and applications in disease modeling.Biochim Biophys Acta Rev Cancer. 2021 Apr;1875(2):188527. doi: 10.1016/j.bbcan.2021.188527. Epub 2021 Feb 26. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33640383 Free PMC article. Review.
-
The Microbiome as a Key Regulator of Female Genital Tract Barrier Function.Front Cell Infect Microbiol. 2021 Dec 17;11:790627. doi: 10.3389/fcimb.2021.790627. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34976864 Free PMC article. Review.
References
-
- Bennett J., Dolin R., Blaser M. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Am. Med. Assoc. 2014;304:2067–2701.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

