International validation of a urinary biomarker panel for identification of active lupus nephritis in children
- PMID: 27590021
- PMCID: PMC5203828
- DOI: 10.1007/s00467-016-3485-3
International validation of a urinary biomarker panel for identification of active lupus nephritis in children
Abstract
Background: Conventional markers of juvenile-onset systemic lupus erythematosus (JSLE) disease activity fail to adequately identify lupus nephritis (LN). While individual novel urine biomarkers are good at detecting LN flares, biomarker panels may improve diagnostic accuracy. The aim of this study was to assess the performance of a biomarker panel to identify active LN in two international JSLE cohorts.
Methods: Novel urinary biomarkers, namely vascular cell adhesion molecule-1 (VCAM-1), monocyte chemoattractant protein 1 (MCP-1), lipocalin-like prostaglandin D synthase (LPGDS), transferrin (TF), ceruloplasmin, alpha-1-acid glycoprotein (AGP) and neutrophil gelatinase-associated lipocalin (NGAL), were quantified in a cross-sectional study that included participants of the UK JSLE Cohort Study (Cohort 1) and validated within the Einstein Lupus Cohort (Cohort 2). Binary logistic regression modelling and receiver operating characteristic curve analysis [area under the curve (AUC)] were used to identify and assess combinations of biomarkers for diagnostic accuracy.
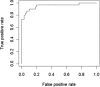
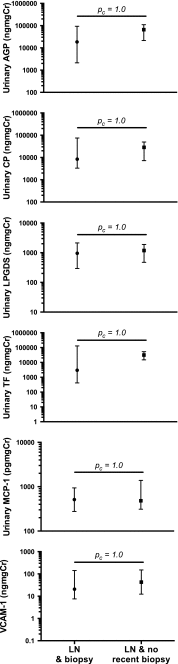
Results: A total of 91 JSLE patients were recruited across both cohorts, of whom 31 (34 %) had active LN and 60 (66 %) had no LN. Urinary AGP, ceruloplasmin, VCAM-1, MCP-1 and LPGDS levels were significantly higher in those patients with active LN than in non-LN patients [all corrected p values (p c) < 0.05] across both cohorts. Urinary TF also differed between patient groups in Cohort 2 (p c = 0.001). Within Cohort 1, the optimal biomarker panel included AGP, ceruloplasmin, LPGDS and TF (AUC 0.920 for active LN identification). These results were validated in Cohort 2, with the same markers resulting in the optimal urine biomarker panel (AUC 0.991).
Conclusion: In two international JSLE cohorts, urinary AGP, ceruloplasmin, LPGDS and TF demonstrate an 'excellent' ability for accurately identifying active LN in children.
Keywords: BILAG; Glomerulonephritis; Lupus nephritis; Systemic lupus erythematosus; Urine biomarkers.
Conflict of interest statement
Compliance with ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Patient assent/consent and parental consent was obtained to participate in the studies. Full ethical approvals were in place from the National Research Ethics Service North West, Liverpool East, UK (reference 06/Q1502/77) and the Institutional Review Board at Einstein-Montefiore (IRB 2000–154). Conflicts of interest There has not been any financial support or other benefits from commercial sources for the work reported on in this manuscript. The authors do not have any financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work. Funding This work was supported by the Alder Hey Children’s Kidney Fund through a training fellowship [UOG10065 to ES]. Lupus UK also provides financial support for co-ordination of the UK JSLE Cohort Study. TR is supported by the Lupus Foundation of America Career Development Award, and the NIH (National Institutes of Health) Loan Repayment Program for Pediatric Research. BG is supported by the Children’s Hospital at Montefiore Young Investigator Award. CP and BG are supported by NIH/NCI 1U19CA179564 and NIH/NCI 1UH2/3TR000933. The funding bodies detailed above were not involved in the design, collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Figures


References
-
- Watson L, Leone V, Pilkington C, Tullus K, Rangaraj S, McDonagh JE, Gardner-Medwin J, Wilkinson N, Riley P, Tizard J, Armon K, Sinha MD, Ioannou Y, Archer N, Bailey K, Davidson J, Baildam EM, Cleary G, McCann LJ, Beresford MW. Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis Rheum. 2012;64(7):2356–2365. doi: 10.1002/art.34410. - DOI - PubMed
-
- Tucker LB, Uribe AG, Fernandez M, Vila LM, McGwin G, Apte M, Fessler BJ, Bastian HM, Reveille JD, Alarcon GS. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case–control study within LUMINA, a multiethnic US cohort (LUMINA LVII) Lupus. 2008;17(4):314–322. doi: 10.1177/0961203307087875. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

