A Lytic Polysaccharide Monooxygenase with Broad Xyloglucan Specificity from the Brown-Rot Fungus Gloeophyllum trabeum and Its Action on Cellulose-Xyloglucan Complexes
- PMID: 27590806
- PMCID: PMC5086550
- DOI: 10.1128/AEM.01768-16
A Lytic Polysaccharide Monooxygenase with Broad Xyloglucan Specificity from the Brown-Rot Fungus Gloeophyllum trabeum and Its Action on Cellulose-Xyloglucan Complexes
Abstract
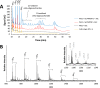
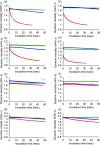
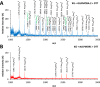
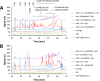
Fungi secrete a set of glycoside hydrolases and lytic polysaccharide monooxygenases (LPMOs) to degrade plant polysaccharides. Brown-rot fungi, such as Gloeophyllum trabeum, tend to have few LPMOs, and information on these enzymes is scarce. The genome of G. trabeum encodes four auxiliary activity 9 (AA9) LPMOs (GtLPMO9s), whose coding sequences were amplified from cDNA. Due to alternative splicing, two variants of GtLPMO9A seem to be produced, a single-domain variant, GtLPMO9A-1, and a longer variant, GtLPMO9A-2, which contains a C-terminal domain comprising approximately 55 residues without a predicted function. We have overexpressed the phylogenetically distinct GtLPMO9A-2 in Pichia pastoris and investigated its properties. Standard analyses using high-performance anion-exchange chromatography-pulsed amperometric detection (HPAEC-PAD) and mass spectrometry (MS) showed that GtLPMO9A-2 is active on cellulose, carboxymethyl cellulose, and xyloglucan. Importantly, compared to other known xyloglucan-active LPMOs, GtLPMO9A-2 has broad specificity, cleaving at any position along the β-glucan backbone of xyloglucan, regardless of substitutions. Using dynamic viscosity measurements to compare the hemicellulolytic action of GtLPMO9A-2 to that of a well-characterized hemicellulolytic LPMO, NcLPMO9C from Neurospora crassa revealed that GtLPMO9A-2 is more efficient in depolymerizing xyloglucan. These measurements also revealed minor activity on glucomannan that could not be detected by the analysis of soluble products by HPAEC-PAD and MS and that was lower than the activity of NcLPMO9C. Experiments with copolymeric substrates showed an inhibitory effect of hemicellulose coating on cellulolytic LPMO activity and did not reveal additional activities of GtLPMO9A-2. These results provide insight into the LPMO potential of G. trabeum and provide a novel sensitive method, a measurement of dynamic viscosity, for monitoring LPMO activity.
Importance: Currently, there are only a few methods available to analyze end products of lytic polysaccharide monooxygenase (LPMO) activity, the most common ones being liquid chromatography and mass spectrometry. Here, we present an alternative and sensitive method based on measurement of dynamic viscosity for real-time continuous monitoring of LPMO activity in the presence of water-soluble hemicelluloses, such as xyloglucan. We have used both these novel and existing analytical methods to characterize a xyloglucan-active LPMO from a brown-rot fungus. This enzyme, GtLPMO9A-2, differs from previously characterized LPMOs in having broad substrate specificity, enabling almost random cleavage of the xyloglucan backbone. GtLPMO9A-2 acts preferentially on free xyloglucan, suggesting a preference for xyloglucan chains that tether cellulose fibers together. The xyloglucan-degrading potential of GtLPMO9A-2 suggests a role in decreasing wood strength at the initial stage of brown rot through degradation of the primary cell wall.
Copyright © 2016 Kojima et al.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

