Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters
- PMID: 27591044
- PMCID: PMC5050440
- DOI: 10.1124/pr.115.012260
Kinase-dependent Regulation of Monoamine Neurotransmitter Transporters
Abstract
Modulation of neurotransmission by the monoamines dopamine (DA), norepinephrine (NE), and serotonin (5-HT) is critical for normal nervous system function. Precise temporal and spatial control of this signaling in mediated in large part by the actions of monoamine transporters (DAT, NET, and SERT, respectively). These transporters act to recapture their respective neurotransmitters after release, and disruption of clearance and reuptake has significant effects on physiology and behavior and has been linked to a number of neuropsychiatric disorders. To ensure adequate and dynamic control of these transporters, multiple modes of control have evolved to regulate their activity and trafficking. Central to many of these modes of control are the actions of protein kinases, whose actions can be direct or indirectly mediated by kinase-modulated protein interactions. Here, we summarize the current state of our understanding of how protein kinases regulate monoamine transporters through changes in activity, trafficking, phosphorylation state, and interacting partners. We highlight genetic, biochemical, and pharmacological evidence for kinase-linked control of DAT, NET, and SERT and, where applicable, provide evidence for endogenous activators of these pathways. We hope our discussion can lead to a more nuanced and integrated understanding of how neurotransmitter transporters are controlled and may contribute to disorders that feature perturbed monoamine signaling, with an ultimate goal of developing better therapeutic strategies.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

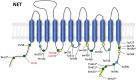
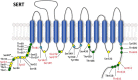
References
-
- Amara SG, editor (1998) Neurotransmitter Transporters, Academic Press, San Diego, CA.
-
- Andreetta F, Barnes NM, Wren PB, Carboni L. (2013) p38 MAP kinase activation does not stimulate serotonin transport in rat brain: Implications for sickness behaviour mechanisms. Life Sci 93:30–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

