On the Stability of BOLD fMRI Correlations
- PMID: 27591147
- PMCID: PMC6248456
- DOI: 10.1093/cercor/bhw265
On the Stability of BOLD fMRI Correlations
Abstract
Measurement of correlations between brain regions (functional connectivity) using blood oxygen level dependent (BOLD) fMRI has proven to be a powerful tool for studying the functional organization of the brain. Recently, dynamic functional connectivity has emerged as a major topic in the resting-state BOLD fMRI literature. Here, using simulations and multiple sets of empirical observations, we confirm that imposed task states can alter the correlation structure of BOLD activity. However, we find that observations of "dynamic" BOLD correlations during the resting state are largely explained by sampling variability. Beyond sampling variability, the largest part of observed "dynamics" during rest is attributable to head motion. An additional component of dynamic variability during rest is attributable to fluctuating sleep state. Thus, aside from the preceding explanatory factors, a single correlation structure-as opposed to a sequence of distinct correlation structures-may adequately describe the resting state as measured by BOLD fMRI. These results suggest that resting-state BOLD correlations do not primarily reflect moment-to-moment changes in cognitive content. Rather, resting-state BOLD correlations may predominantly reflect processes concerned with the maintenance of the long-term stability of the brain's functional organization.
Keywords: BOLD fMRI; dynamics; functional connectivity; nonstationarity; resting state.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
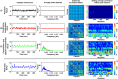
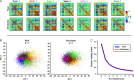
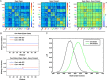


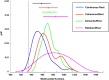
References
-
- Bishop GH. 1932. Cyclic changes in excitability of the optic pathway of the rabbit. Am J Physiol. 103:213–224.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

