Examining Plant Physiological Responses to Climate Change through an Evolutionary Lens
- PMID: 27591186
- PMCID: PMC5047093
- DOI: 10.1104/pp.16.00793
Examining Plant Physiological Responses to Climate Change through an Evolutionary Lens
Abstract
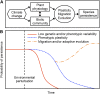
Integrating knowledge from physiological ecology, evolutionary biology, phylogenetics, and paleobiology provides novel insights into factors driving plant physiological responses to both past and future climate change.
Figures


References
-
- Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR, Geber MA, Evans AS, Dawson TE, et al. (2000) The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience 50: 979–995
-
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 - PubMed
-
- Aitken SN, Whitlock MC (2013) Assisted gene flow to facilitate local adaptation to climate change. Annu Rev Ecol Evol Syst 44: 367–388
-
- Alberton O, Kuyper TW, Gorissen A (2007) Competition for nitrogen between Pinus sylvestris and ectomycorrhizal fungi generates potential for negative feedback under elevated CO2. Plant Soil 296: 159–172
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

