Elevated Temperature and CO2 Stimulate Late-Season Photosynthesis But Impair Cold Hardening in Pine
- PMID: 27591187
- PMCID: PMC5047089
- DOI: 10.1104/pp.16.00753
Elevated Temperature and CO2 Stimulate Late-Season Photosynthesis But Impair Cold Hardening in Pine
Abstract
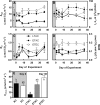
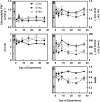
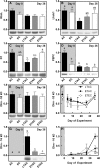
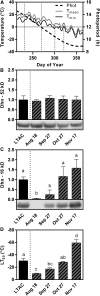
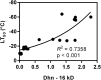
Rising global temperature and CO2 levels may sustain late-season net photosynthesis of evergreen conifers but could also impair the development of cold hardiness. Our study investigated how elevated temperature, and the combination of elevated temperature with elevated CO2, affected photosynthetic rates, leaf carbohydrates, freezing tolerance, and proteins involved in photosynthesis and cold hardening in Eastern white pine (Pinus strobus). We designed an experiment where control seedlings were acclimated to long photoperiod (day/night 14/10 h), warm temperature (22°C/15°C), and either ambient (400 μL L-1) or elevated (800 μmol mol-1) CO2, and then shifted seedlings to growth conditions with short photoperiod (8/16 h) and low temperature/ambient CO2 (LTAC), elevated temperature/ambient CO2 (ETAC), or elevated temperature/elevated CO2 (ETEC). Exposure to LTAC induced down-regulation of photosynthesis, development of sustained nonphotochemical quenching, accumulation of soluble carbohydrates, expression of a 16-kD dehydrin absent under long photoperiod, and increased freezing tolerance. In ETAC seedlings, photosynthesis was not down-regulated, while accumulation of soluble carbohydrates, dehydrin expression, and freezing tolerance were impaired. ETEC seedlings revealed increased photosynthesis and improved water use efficiency but impaired dehydrin expression and freezing tolerance similar to ETAC seedlings. Sixteen-kilodalton dehydrin expression strongly correlated with increases in freezing tolerance, suggesting its involvement in the development of cold hardiness in P. strobus Our findings suggest that exposure to elevated temperature and CO2 during autumn can delay down-regulation of photosynthesis and stimulate late-season net photosynthesis in P. strobus seedlings. However, this comes at the cost of impaired freezing tolerance. Elevated temperature and CO2 also impaired freezing tolerance. However, unless the frequency and timing of extreme low-temperature events changes, this is unlikely to increase risk of freezing damage in P. strobus seedlings.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Adams WW III, Zarter CR, Ebbert V, Demmig-Adams B (2004) Photoprotective strategies of overwintering evergreens. Bioscience 54: 41–49
-
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165: 351–371 - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 - PubMed
-
- Angelcheva L, Mishra Y, Antti H, Kjellsen TD, Funk C, Strimbeck RG, Schröder WP (2014) Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol 204: 545–555 - PubMed
-
- Basler D, Körner C (2012) Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric For Meteorol 165: 73–81
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

