Preclinical rationale for TGF-β inhibition as a therapeutic target for the treatment of myelofibrosis
- PMID: 27592389
- PMCID: PMC5778911
- DOI: 10.1016/j.exphem.2016.08.007
Preclinical rationale for TGF-β inhibition as a therapeutic target for the treatment of myelofibrosis
Abstract
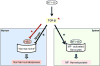
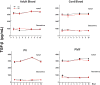
To assess the role of abnormal transforming growth factor-beta (TGF-β) signaling in the pathogenesis of primary myelofibrosis (PMF), the effects of the TGF-β receptor-1 kinase inhibitor SB431542 on ex vivo expansion of hematopoietic cells in cultures from patients with JAK2V617+-polycythemia vera (PV) or PMF (JAK2V617F+, CALRpQ365f+, or unknown) and from normal sources (adult blood, AB, or cord blood, CB) were compared. In cultures of normal sources, SB431542 significantly increased by 2.5-fold the number of progenitor cells generated by days 1-2 (CD34+) and 6 (colony-forming cells) (CB) and that of precursor cells, mostly immature erythroblasts, by days 14-17 (AB and CB). In cultures of JAK2V617F+-PV, SB431542 increased by twofold the numbers of progenitor cells by day 10 and had no effect on that of precursors cells by days 12-17 (∼fourfold increase in all cases). In contrast, SB431542 had no effect on the number of either progenitor or precursor cells in cultures of JAK2V617F+ and CALR pQ365fs+ PMF. These ontogenetic- and disease-specific effects were associated with variegation in the ability of SB431542 to induce CD34+ cells from AB (increased), CB (decreased), or PV and PMF (unaffected) into cycle and erythroblasts in proliferation (increased for AB and PV and unaffected for CB and PMF). Differences in expansion of erythroblasts from AB, CB, and PV were associated with differences in activation of TGF-β signaling (SHCY317, SMAD2S245/250/255, and SMAD1S/S/SMAD5S/S/SMAD8S/S) detectable in these cells by phosphoproteomic profiling. In conclusion, treatment with TGF-β receptor-1 kinase inhibitors may reactivate normal hematopoiesis in PMF patients, providing a proliferative advantage over the unresponsive malignant clone.
Copyright © 2016 ISEH - International Society for Experimental Hematology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures










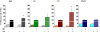


References
-
- Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer E-M. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. - PubMed
-
- Campanelli R, Rosti V, Villani L, et al. Evaluation of the bioactive and total transforming growth factor β1 levels in primary myelofibrosis. Cytokine. 2011;53:100–106. - PubMed
-
- Chagraoui H, Komura E, Tulliez M, Giraudier S, Vainchenker W, Wendling F. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100:3495–3503. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

