Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial
- PMID: 27592805
- PMCID: PMC5587154
- DOI: 10.1016/S1470-2045(16)30364-3
Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial
Abstract
Background: Merkel cell carcinoma is a rare, aggressive skin cancer with poor prognosis in patients with advanced disease. Current standard care uses various cytotoxic chemotherapy regimens, but responses are seldom durable. Tumour oncogenesis is linked to Merkel cell polyomavirus integration and ultraviolet-radiation-induced mutations, providing rationale for treatment with immunotherapy antibodies that target the PD-L1/PD-1 pathway. We assessed treatment with avelumab, an anti-PD-L1 monoclonal antibody, in patients with stage IV Merkel cell carcinoma that had progressed after cytotoxic chemotherapy.
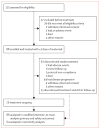
Methods: In this multicentre, international, prospective, single-group, open-label, phase 2 trial, patients with stage IV chemotherapy-refractory, histologically confirmed Merkel cell carcinoma (aged ≥18 years) were enrolled from 35 cancer treatment centres and academic hospitals in North America, Europe, Australia, and Asia. Key eligibility criteria were an ECOG performance status of 0 or 1, measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, adequate haematological, hepatic, and renal function, and immune-competent status (patients with HIV, immunosuppression, haematological malignancies, and previous organ transplantation were excluded). Patient selection was not based on PD-L1 expression or Merkel cell polyomavirus status. Collection of biopsy material or use of archival tissue for these assessments was mandatory. Avelumab was given intravenously at a dose of 10 mg/kg every 2 weeks. The primary endpoint was confirmed objective response (complete response or partial response) assessed according to RECIST version 1.1 by an independent review committee. Safety and clinical activity were assessed in all patients who received at least one dose of study drug (the modified intention-to-treat population). This trial is registered with ClinicalTrials.gov as NCT02155647.
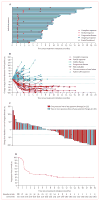
Findings: Between July 25, 2014, and Sept 3, 2015, 88 patients were enrolled and received at least one dose of avelumab. Patients were followed up for a median of 10·4 months (IQR 8·6-13·1). The proportion of patients who achieved an objective response was 28 (31·8% [95·9% CI 21·9-43·1]) of 88 patients, including eight complete responses and 20 partial responses. Responses were ongoing in 23 (82%) of 28 patients at the time of analysis. Five grade 3 treatment-related adverse events occurred in four (5%) patients: lymphopenia in two patients, blood creatine phosphokinase increase in one patient, aminotransferase increase in one patient, and blood cholesterol increase in one patient; there were no treatment-related grade 4 adverse events or treatment-related deaths. Serious treatment-related adverse events were reported in five patients (6%): enterocolitis, infusion-related reaction, aminotransferases increased, chondrocalcinosis, synovitis, and interstitial nephritis (n=1 each).
Interpretation: Avelumab was associated with durable responses, most of which are still ongoing, and was well tolerated; hence, avelumab represents a new therapeutic option for advanced Merkel cell carcinoma.
Funding: Merck KGaA, Darmstadt, Germany.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
HLK reports personal fees from Alkermes, Amgen, EMD Serono, Prometheus, and Sanofi; non-financial support from Merck; grants from Bristol-Myers Squibb, outside the submitted work. OH reports personal fees from Merck, outside the submitted work. PT reports personal fees from Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, Novartis, and GlaxoSmithKline, outside the submitted work. SPD’A reports personal fees from EMD Serono and Amgen, outside the submitted work. CL reports personal fees from Novartis, Bristol-Myers Squibb, Roche Glycart, Amgen, and Merck Sharp & Dohme, outside the submitted work. MM reports personal fees from AstraZeneca, Novartis, Pfizer, Celgene, and NeoPharm, outside the submitted work. KDL reports institutional research funding from EMD Serono, outside the submitted work. KC is an employee of EMD Serono and holds stock in Bristol-Myers Squibb. LM is an employee of EMD Serono. AvH is an employee and stockholder at Merck KGaA. J-MC is an employee of EMD Serono. PN has been reimbursed for travel, accommodation, or expenses from EMD Serono, outside the submitted work. All other authors declare no competing interests.
Figures

Comment in
-
Checkpoint inhibitors: a new standard of care for advanced Merkel cell carcinoma?Lancet Oncol. 2016 Oct;17(10):1337-1339. doi: 10.1016/S1470-2045(16)30441-7. Epub 2016 Sep 1. Lancet Oncol. 2016. PMID: 27592804 No abstract available.
-
Immune Checkpoint Inhibition in Cancers that Affect the Head and Neck.Int J Radiat Oncol Biol Phys. 2017 Aug 1;98(5):969-973. doi: 10.1016/j.ijrobp.2017.03.003. Epub 2017 Jul 10. Int J Radiat Oncol Biol Phys. 2017. PMID: 28721906 No abstract available.
References
-
- Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–41. - PubMed
-
- Lebbé C, Becker JC, Grob JJ, et al. Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2396–403. - PubMed
-
- Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015;81:802–06. - PubMed
-
- Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014;150:864–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

