Modulating Neuronal Competition Dynamics in the Dentate Gyrus to Rejuvenate Aging Memory Circuits
- PMID: 27593178
- PMCID: PMC5033725
- DOI: 10.1016/j.neuron.2016.08.009
Modulating Neuronal Competition Dynamics in the Dentate Gyrus to Rejuvenate Aging Memory Circuits
Abstract
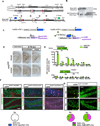
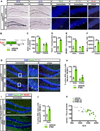
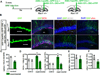
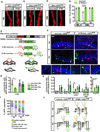
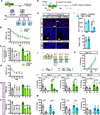
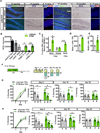
The neural circuit mechanisms underlying the integration and functions of adult-born dentate granule cell (DGCs) are poorly understood. Adult-born DGCs are thought to compete with mature DGCs for inputs to integrate. Transient genetic overexpression of a negative regulator of dendritic spines, Kruppel-like factor 9 (Klf9), in mature DGCs enhanced integration of adult-born DGCs and increased NSC activation. Reversal of Klf9 overexpression in mature DGCs restored spines and activity and reset neuronal competition dynamics and NSC activation, leaving the DG modified by a functionally integrated, expanded cohort of age-matched adult-born DGCs. Spine elimination by inducible deletion of Rac1 in mature DGCs increased survival of adult-born DGCs without affecting proliferation or DGC activity. Enhanced integration of adult-born DGCs transiently reorganized adult-born DGC local afferent connectivity and promoted global remapping in the DG. Rejuvenation of the DG by enhancing integration of adult-born DGCs in adulthood, middle age, and aging enhanced memory precision.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Adult neurogenesis: Encouraging integration.Nat Rev Neurosci. 2016 Nov;17(11):669. doi: 10.1038/nrn.2016.136. Epub 2016 Sep 22. Nat Rev Neurosci. 2016. PMID: 27654861 No abstract available.
References
-
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

