Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure
- PMID: 27595324
- PMCID: PMC5053881
- DOI: 10.1038/nm.4177
Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure
Erratum in
-
Corrigendum: Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure.Nat Med. 2017 Apr 7;23(4):526. doi: 10.1038/nm0417-526a. Nat Med. 2017. PMID: 28388609 No abstract available.
-
Corrigendum: Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure.Nat Med. 2017 Dec 7;23(12):1499. doi: 10.1038/nm1217-1499a. Nat Med. 2017. PMID: 29216039 Free PMC article.
Abstract
The absence of a gold standard to determine when antibiotics induce a sterilizing cure has confounded the development of new approaches to treat pulmonary tuberculosis (PTB). We detected positron emission tomography and computerized tomography (PET-CT) imaging response patterns consistent with active disease, along with the presence of Mycobacterium tuberculosis (MTB) mRNA in sputum and bronchoalveolar lavage samples, in a substantial proportion of adult, HIV-negative patients with PTB after a standard 6-month treatment plus 1 year follow-up, including patients with a durable cure and others who later developed recurrent disease. The presence of MTB mRNA in the context of nonresolving and intensifying lesions on PET-CT images might indicate ongoing transcription, suggesting that even apparently curative treatment for PTB may not eradicate all of the MTB bacteria in most patients. This suggests an important complementary role for the immune response in maintaining a disease-free state. Sterilizing drugs or host-directed therapies, and better treatment response markers, are probably needed for the successful development of improved and shortened PTB-treatment strategies.
Conflict of interest statement
statement. We have no competing financial interest to declare.
Figures
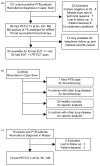
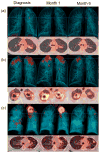

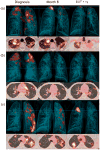
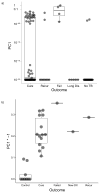
Comment in
-
A broader spectrum of tuberculosis.Nat Med. 2016 Oct 6;22(10):1076-1077. doi: 10.1038/nm.4186. Nat Med. 2016. PMID: 27711059 No abstract available.
References
-
- WHO. Global Tuberculosis Report. 2015;20
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

