VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors
- PMID: 27595385
- PMCID: PMC5519826
- DOI: 10.1038/nn.4377
VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors
Abstract
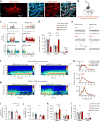
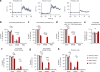
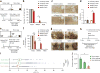
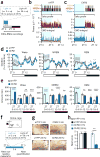
Dopaminergic ventral tegmental area (VTA) neurons are critically involved in a variety of behaviors that rely on heightened arousal, but whether they directly and causally control the generation and maintenance of wakefulness is unknown. We recorded calcium activity using fiber photometry in freely behaving mice and found arousal-state-dependent alterations in VTA dopaminergic neurons. We used chemogenetic and optogenetic manipulations together with polysomnographic recordings to demonstrate that VTA dopaminergic neurons are necessary for arousal and that their inhibition suppresses wakefulness, even in the face of ethologically relevant salient stimuli. Nevertheless, before inducing sleep, inhibition of VTA dopaminergic neurons promoted goal-directed and sleep-related nesting behavior. Optogenetic stimulation, in contrast, initiated and maintained wakefulness and suppressed sleep and sleep-related nesting behavior. We further found that different projections of VTA dopaminergic neurons differentially modulate arousal. Collectively, our findings uncover a fundamental role for VTA dopaminergic circuitry in the maintenance of the awake state and ethologically relevant sleep-related behaviors.
Figures


Comment in
-
Too bored to stay awake.Nat Neurosci. 2016 Sep 27;19(10):1274-6. doi: 10.1038/nn.4383. Nat Neurosci. 2016. PMID: 27669985 No abstract available.
References
-
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl.) 2007;191:433–437. - PubMed
-
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience. Brain Res. Brain Res. Rev. 1998;28:309–369. - PubMed
-
- Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. - PubMed
-
- Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

