Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells
- PMID: 27596381
- PMCID: PMC5011778
- DOI: 10.1038/srep32835
Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells
Abstract
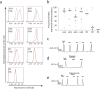
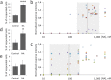
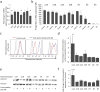
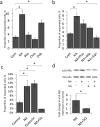
Sympathetic neurons are important components of mesenchymal stem cells (MSCs) niche and noradrenaline regulates biological activities of these cells. Here we examined the mechanisms of regulation of MSCs responsiveness to noradrenaline. Using flow cytometry, we demonstrated that α1A adrenergic receptors isoform was the most abundant in adipose tissue-derived MSCs. Using calcium imaging in single cells, we demonstrated that only 6.9 ± 0.8% of MSCs responded to noradrenaline by intracellular calcium release. Noradrenaline increases MSCs sensitivity to catecholamines in a transitory mode. Within 6 hrs after incubation with noradrenaline the proportion of cells responding by Ca(2+) release to the fresh noradrenaline addition has doubled but declined to the baseline after 24 hrs. Increased sensitivity was due to the elevated quantities of α1A-adrenergic receptors on MSCs. Such elevation depended on the stimulation of β-adrenergic receptors and adenylate cyclase activation. The data for the first time clarify mechanisms of regulation of MSCs sensitivity to noradrenaline.
Figures
References
-
- Phinney D. G. & Prockop D. J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 25, 2896–2902 (2007). - PubMed
-
- Rehman J. et al.. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 (2004). - PubMed
-
- Lee S. M., Lee S. C. & Kim S. J. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res 188, 280–289 (2014). - PubMed
-
- Wei X. et al.. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells 27, 478–488 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

