Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: a systematic review and meta-analysis
- PMID: 27596383
- PMCID: PMC5011653
- DOI: 10.1038/srep32649
Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: a systematic review and meta-analysis
Abstract
Alpha-glucosidase inhibitors (AGIs) was reported to be associated with several rare adverse hepatic events, but with inconsistent results. We aimed to investigate the risk of hepatotoxicity associated with the use of AGIs in patients with type 2 diabetes mellitus (T2DM), and performed a systematic review and meta-analysis. Fourteen studies (n = 2881) were eligible, all of which were RCTs. Meta-analysis of data regarding elevation of more than 3-fold the upper limit of normal (ULN) of AST and ALT showed statistically significant differences between AGIs treatment versus control (OR 6.86, 95% CI 2.50 to 18.80; OR 6.48, 95% CI 2.40 to 17.49). Subgroup analyses of elevation of more than 1.8-fold ULN of AST and ALT by dose of AGIs showed differential effects on AST and ALT (AST: OR 0.38 vs 7.31, interaction P = 0.003; ALT: OR 0.32 vs 4.55, interaction p = 0.02). Meta-analysis showed that AGIs might increase the risk of hepatotoxicity, and higher dose appeared to be associated with higher risk of hepatotoxicity. However, the evidence is limited with surrogate measures (i.e. ALT and AST), and no clinically important adverse events were observed.
Figures
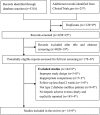




Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
Cited by
-
Gene ontology enrichment analysis of α-amylase inhibitors from Duranta repens in diabetes mellitus.J Diabetes Metab Disord. 2020 Jun 7;19(2):735-747. doi: 10.1007/s40200-020-00554-9. eCollection 2020 Dec. J Diabetes Metab Disord. 2020. PMID: 33520800 Free PMC article.
-
Designing Potent α-Glucosidase Inhibitors: A Synthesis and QSAR Modeling Approach for Biscoumarin Derivatives.ACS Omega. 2023 Jul 11;8(29):26340-26350. doi: 10.1021/acsomega.3c02868. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521599 Free PMC article.
-
Hypoglycemic activity of the ethyl acetate extract from Smilax glabra Roxb in mice: Biochemical and histopathological studies.Iran J Basic Med Sci. 2020 Dec;23(12):1558-1564. doi: 10.22038/ijbms.2020.46658.10763. Iran J Basic Med Sci. 2020. PMID: 33489029 Free PMC article.
-
Genomic analysis and in vivo efficacy of Pediococcus acidilactici as a potential probiotic to prevent hyperglycemia, hypercholesterolemia and gastrointestinal infections.Sci Rep. 2022 Nov 28;12(1):20429. doi: 10.1038/s41598-022-24791-5. Sci Rep. 2022. PMID: 36443433 Free PMC article.
-
Factors That Influence Pancreatic Beta Cell Function and Insulin Resistance in Newly Diagnosed Type 2 Diabetes Patients: A Sub-Analysis of the MARCH Trial.Diabetes Ther. 2018 Apr;9(2):743-752. doi: 10.1007/s13300-018-0393-5. Epub 2018 Mar 9. Diabetes Ther. 2018. PMID: 29524187 Free PMC article.
References
-
- Stephen P. & Clissold C. E. Acarbose. Drugs. 25, 214–243 (1988). - PubMed
-
- Silvio E., Inzucchi R. M. B. et al.. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 38, 140–149, doi: 10.2337/dc14-2441/-/DC1 (2015). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

