Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: a systematic review and meta-analysis
- PMID: 27596383
- PMCID: PMC5011653
- DOI: 10.1038/srep32649
Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: a systematic review and meta-analysis
Abstract
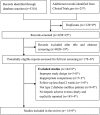
Alpha-glucosidase inhibitors (AGIs) was reported to be associated with several rare adverse hepatic events, but with inconsistent results. We aimed to investigate the risk of hepatotoxicity associated with the use of AGIs in patients with type 2 diabetes mellitus (T2DM), and performed a systematic review and meta-analysis. Fourteen studies (n = 2881) were eligible, all of which were RCTs. Meta-analysis of data regarding elevation of more than 3-fold the upper limit of normal (ULN) of AST and ALT showed statistically significant differences between AGIs treatment versus control (OR 6.86, 95% CI 2.50 to 18.80; OR 6.48, 95% CI 2.40 to 17.49). Subgroup analyses of elevation of more than 1.8-fold ULN of AST and ALT by dose of AGIs showed differential effects on AST and ALT (AST: OR 0.38 vs 7.31, interaction P = 0.003; ALT: OR 0.32 vs 4.55, interaction p = 0.02). Meta-analysis showed that AGIs might increase the risk of hepatotoxicity, and higher dose appeared to be associated with higher risk of hepatotoxicity. However, the evidence is limited with surrogate measures (i.e. ALT and AST), and no clinically important adverse events were observed.
Figures




References
-
- Stephen P. & Clissold C. E. Acarbose. Drugs. 25, 214–243 (1988). - PubMed
-
- Silvio E., Inzucchi R. M. B. et al.. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 38, 140–149, doi: 10.2337/dc14-2441/-/DC1 (2015). - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

