SMARTphone-based, early cardiac REHABilitation in patients with acute coronary syndromes [SMART-REHAB Trial]: a randomized controlled trial protocol
- PMID: 27596569
- PMCID: PMC5011930
- DOI: 10.1186/s12872-016-0356-6
SMARTphone-based, early cardiac REHABilitation in patients with acute coronary syndromes [SMART-REHAB Trial]: a randomized controlled trial protocol
Abstract
Background: There are well-documented treatment gaps in secondary prevention of coronary heart disease and no clear guidelines to assist early physical activity after acute coronary syndromes (ACS). Smartphone technology may provide an innovative platform to close these gaps. This paper describes the study design of a randomized controlled trial assessing whether a smartphone-based secondary prevention program can facilitate early physical activity and improve cardiovascular health in patients with ACS.
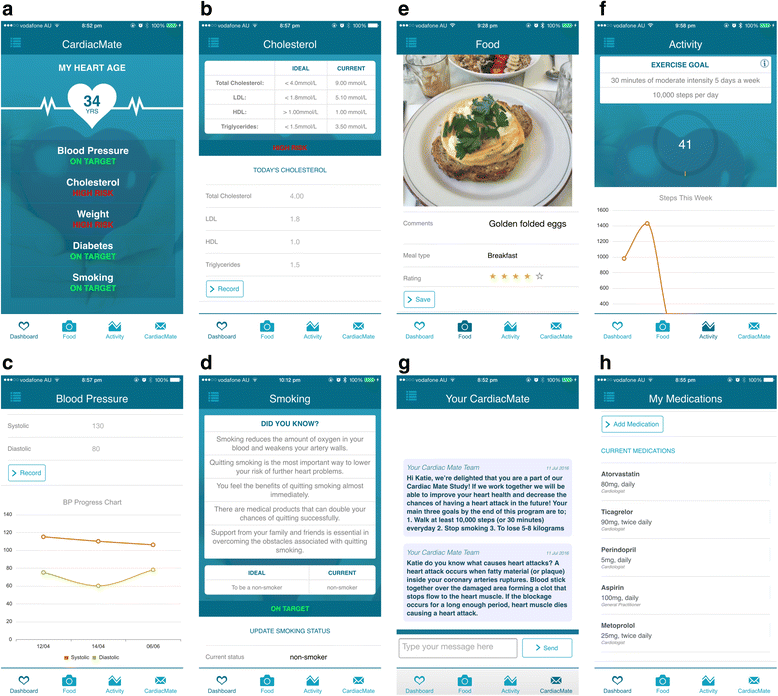
Methods: We have developed a multi-faceted, patient-centred smartphone-based secondary prevention program emphasizing early physical activity with a graduated walking program initiated on discharge from ACS admission. The program incorporates; physical activity tracking through the smartphone's accelerometer with interactive feedback and goal setting; a dynamic dashboard to review and optimize cardiovascular risk factors; educational messages delivered twice weekly; a photographic food diary; pharmacotherapy review; and support through a short message service. The primary endpoint of the trial is change in exercise capacity, as measured by the change in six-minute walk test distance at 8-weeks when compared to baseline. Secondary endpoints include improvements in cardiovascular risk factor status, psychological well-being and quality of life, medication adherence, uptake of cardiac rehabilitation and re-hospitalizations.
Discussion: This randomized controlled trial will use a smartphone-phone based secondary prevention program to emphasize early physical activity post-ACS. It will provide evidence regarding the feasibility and utility of this innovative platform in closing the treatment gaps in secondary prevention.
Trial registration: The trial was retrospectively registered in the Australian New Zealand Clinical Trials Registry (ANZCTR) on April 4, 2016. The registration number is ACTRN12616000426482 .
Keywords: Acute coronary syndromes; Cardiac rehabilitation; Secondary prevention; Smartphone application; mhealth.
Figures

References
-
- The Top 10 Causes of Death. Fact Sheet Number 310. [http://www.who.int/mediacentre/factsheets/fs310/en/index.html]. Accessed 31 Aug 2016.
-
- Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American heart association and American college of cardiology foundation endorsed by the world heart federation and the preventive cardiovascular nurses association. J Am Coll Cardiol. 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

