Regulatory T cells in allergic diseases
- PMID: 27596705
- PMCID: PMC5023156
- DOI: 10.1016/j.jaci.2016.06.003
Regulatory T cells in allergic diseases
Abstract
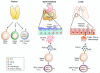
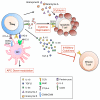
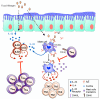
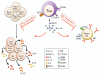
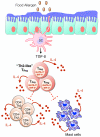
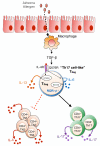
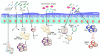
The pathogenesis of allergic diseases entails an ineffective tolerogenic immune response to allergens. Regulatory T (Treg) cells play a key role in sustaining immune tolerance to allergens, yet mechanisms by which Treg cells fail to maintain tolerance in patients with allergic diseases are not well understood. We review current concepts and established mechanisms regarding how Treg cells regulate different components of allergen-triggered immune responses to promote and maintain tolerance. We will also discuss more recent advances that emphasize the "dual" functionality of Treg cells in patients with allergic diseases: how Treg cells are essential in promoting tolerance to allergens but also how a proallergic inflammatory environment can skew Treg cells toward a pathogenic phenotype that aggravates and perpetuates disease. These advances highlight opportunities for novel therapeutic strategies that aim to re-establish tolerance in patients with chronic allergic diseases by promoting Treg cell stability and function.
Keywords: Asthma; IL-4; T(H)2 cells; food allergy; forkhead box P3; regulatory T cells.
Copyright © 2016 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291–307. quiz308. - PubMed
-
- Loftus PA, Wise SK. Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg. 2016;24:245–9. - PubMed
-
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2014;16:45–56. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125:S116–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

