Development of an in vivo bone fatigue damage model using axial compression of the rabbit forelimb
- PMID: 27596952
- PMCID: PMC5862430
- DOI: 10.1016/j.jbiomech.2016.08.020
Development of an in vivo bone fatigue damage model using axial compression of the rabbit forelimb
Abstract
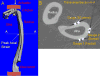
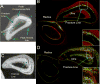
Many nontraumatic fractures seen clinically in patients with metabolic bone disorders or on antiresorptive treatment show an increased incidence of microdamage accumulation and impaired intracortical remodeling. However, the lack of basal remodeling and Haversian bone in rodents limits their translatability in studying bone damage repair mechanisms. The work presented here demonstrates the development of the forelimb loading model in rabbits, the smallest mammal with intracortical Haversian remodeling. The forelimbs of post-mortem female New Zealand white rabbits were loaded in axial end compression to determine their basic monotonic and fatigue properties. Following time zero characterization, stress fractures were created in vivo and animals were allowed to recover for a period of two to five weeks. The rabbit forelimb when loaded in axial compression demonstrates a consistent mid-diaphyseal fracture location characterized by a local mixed compression-bending loading environment. Forelimb apparent stiffness, when fatigue loaded, demonstrates a progressive increase until macrocrack formation, at which time apparent stiffness rapidly declines until failure. Stress fractures in the rabbit ulna display robust periosteal expansion and woven bone formation two weeks following fracture. Subsequent healing at five weeks post-fracture is marked by woven bone densification, resorption and intracortical remodeling along the stress fracture line. The rabbit forelimb fatigue model is a promising new platform by which bone׳s response to damage may be studied.
Keywords: Bone damage; Bone fatigue; Bone mechanics; In vivo forelimb loading; Rabbit ulna.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

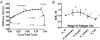

Similar articles
-
An animal trial to study damage and repair in ovariectomized rabbits.J Biomech. 2020 Jul 17;108:109866. doi: 10.1016/j.jbiomech.2020.109866. Epub 2020 Jun 20. J Biomech. 2020. PMID: 32635993 Free PMC article.
-
Intracortical remodeling in adult rat long bones after fatigue loading.Bone. 1998 Sep;23(3):275-81. doi: 10.1016/s8756-3282(98)00104-5. Bone. 1998. PMID: 9737350
-
In vivo fatigue loading of the rat ulna induces both bone formation and resorption and leads to time-related changes in bone mechanical properties and density.J Orthop Res. 2002 Jul;20(4):764-71. doi: 10.1016/S0736-0266(01)00161-9. J Orthop Res. 2002. PMID: 12168665
-
Multiscale computational and experimental approaches to elucidate bone and ligament mechanobiology using the ulna-radius-interosseous membrane construct as a model system.Technol Health Care. 2012;20(5):363-78. doi: 10.3233/THC-2012-0686. Technol Health Care. 2012. PMID: 23079942 Review.
-
Remodeling and the repair of fatigue damage.Calcif Tissue Int. 1993;53 Suppl 1:S75-80; discussion S80-1. doi: 10.1007/BF01673407. Calcif Tissue Int. 1993. PMID: 8275384 Review.
Cited by
-
VEGFA From Early Osteoblast Lineage Cells (Osterix+) Is Required in Mice for Fracture Healing.J Bone Miner Res. 2019 Sep;34(9):1690-1706. doi: 10.1002/jbmr.3755. Epub 2019 Aug 1. J Bone Miner Res. 2019. PMID: 31081125 Free PMC article.
-
Ablation of Proliferating Osteoblast Lineage Cells After Fracture Leads to Atrophic Nonunion in a Mouse Model.J Bone Miner Res. 2021 Nov;36(11):2243-2257. doi: 10.1002/jbmr.4424. Epub 2021 Sep 7. J Bone Miner Res. 2021. PMID: 34405443 Free PMC article.
-
An animal trial to study damage and repair in ovariectomized rabbits.J Biomech. 2020 Jul 17;108:109866. doi: 10.1016/j.jbiomech.2020.109866. Epub 2020 Jun 20. J Biomech. 2020. PMID: 32635993 Free PMC article.
-
Reconstruction of Load-Bearing Segmental Bone Defects Using Carbonate Apatite Honeycomb Blocks.ACS Mater Au. 2023 Apr 26;3(4):321-336. doi: 10.1021/acsmaterialsau.3c00008. eCollection 2023 Jul 12. ACS Mater Au. 2023. PMID: 38090126 Free PMC article.
-
Inferring locomotor behaviours in Miocene New World monkeys using finite element analysis, geometric morphometrics and machine-learning classification techniques applied to talar morphology.J R Soc Interface. 2018 Sep 26;15(146):20180520. doi: 10.1098/rsif.2018.0520. J R Soc Interface. 2018. PMID: 30257926 Free PMC article.
References
-
- Allen MR, Burr DB. Bisphosphonates effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone. 2010;49:56–65. - PubMed
-
- Baumann AP, Aref MW, Turnbull TL, Robling AG, Niebur GL, Allen MR, Roeder RK. Development of an in vivo rabbit ulnar loading model. Bone. 2015;75:55–61. - PubMed
-
- Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone. 1998;23:275–281. - PubMed
-
- Boyce TM, Fyhrie DP, Glotkowski MC, Radin EL, Schaffler MB. Damage type and strain mode associations in human compact bone bending fatigue. Journal of Bone and Joint Surgery. 1998;16:322–329. - PubMed
-
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo atigue microdamage. Journal of Biomechanics. 1985;18:189–200. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

