Food-induced changes of lipids in rat neuronal tissue visualized by ToF-SIMS imaging
- PMID: 27596988
- PMCID: PMC5011716
- DOI: 10.1038/srep32797
Food-induced changes of lipids in rat neuronal tissue visualized by ToF-SIMS imaging
Abstract
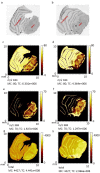
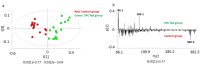
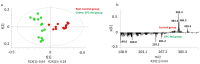
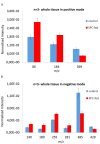
Time of flight secondary ion mass spectrometry (ToF-SIMS) was used to image the lipid localization in brain tissue sections from rats fed specially processed cereals (SPC). An IonTof 5 instrument equipped with a Bi cluster ion gun was used to analyze the tissue sections. Data from 15 brain samples from control and cereal-fed rats were recorded and exported to principal components analysis (PCA). The data clearly show changes of certain lipids in the brain following cereal feeding. PCA score plots show a good separation in lipid distribution between the control and the SPC-fed group. The loadings plot reveal that the groups separated mainly due to changes in cholesterol, vitamin E and c18:2, c16:0 fatty acid distribution as well as some short chain monocarboxylic fatty acid compositions. These insights relate to the working mechanism of SPC as a dietary supplement. SPC is thought to activate antisecretory factor (AF), an endogenous protein with regulatory function for inflammation and fluid secretion. These data provide insights into lipid content in brain following SPC feeding and suggest a relation to activating AF.
Figures
Similar articles
-
Mass spectrometric profiling of lipids in intestinal tissue from rats fed cereals processed for medical conditions.Biointerphases. 2016 Jun 11;11(2):02A310. doi: 10.1116/1.4939599. Biointerphases. 2016. PMID: 26753787
-
Lipid mapping of colonic mucosa by cluster TOF-SIMS imaging and multivariate analysis in cftr knockout mice.J Lipid Res. 2010 Oct;51(10):3034-45. doi: 10.1194/jlr.M008870. Epub 2010 Jul 8. J Lipid Res. 2010. PMID: 20616379 Free PMC article.
-
Localization of lipids in the aortic wall with imaging TOF-SIMS.Biochim Biophys Acta. 2007 Feb;1771(2):185-95. doi: 10.1016/j.bbalip.2006.12.003. Epub 2006 Dec 19. Biochim Biophys Acta. 2007. PMID: 17240191
-
Imaging lipids with secondary ion mass spectrometry.Biochim Biophys Acta. 2014 Aug;1841(8):1108-19. doi: 10.1016/j.bbalip.2014.03.003. Epub 2014 Mar 18. Biochim Biophys Acta. 2014. PMID: 24657337 Review.
-
Lipid imaging with cluster time-of-flight secondary ion mass spectrometry.Anal Bioanal Chem. 2009 Jan;393(1):31-5. doi: 10.1007/s00216-008-2367-3. Epub 2008 Sep 7. Anal Bioanal Chem. 2009. PMID: 18777109 Review.
Cited by
-
Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis.Theranostics. 2022 Jan 1;12(3):1220-1246. doi: 10.7150/thno.65427. eCollection 2022. Theranostics. 2022. PMID: 35154484 Free PMC article.
-
Zinc Deficiency Leads to Lipid Changes in Drosophila Brain Similar to Cognitive-Impairing Drugs: An Imaging Mass Spectrometry Study.Chembiochem. 2020 Oct 1;21(19):2755-2758. doi: 10.1002/cbic.202000197. Epub 2020 Jun 16. Chembiochem. 2020. PMID: 32402134 Free PMC article.
-
Observation of endoplasmic reticulum tubules via TOF-SIMS tandem mass spectrometry imaging of transfected cells.Biointerphases. 2018 Feb 26;13(3):03B409. doi: 10.1116/1.5019736. Biointerphases. 2018. PMID: 29482330 Free PMC article.
-
Antisecretory Factor-Mediated Inhibition of Cell Volume Dynamics Produces Antitumor Activity in Glioblastoma.Mol Cancer Res. 2018 May;16(5):777-790. doi: 10.1158/1541-7786.MCR-17-0413. Epub 2018 Feb 5. Mol Cancer Res. 2018. PMID: 29431617 Free PMC article.
-
Restoring Oat Nanoparticles Mediated Brain Memory Function of Mice Fed Alcohol by Sorting Inflammatory Dectin-1 Complex Into Microglial Exosomes.Small. 2022 Feb;18(6):e2105385. doi: 10.1002/smll.202105385. Epub 2021 Dec 13. Small. 2022. PMID: 34897972 Free PMC article.
References
-
- Johansson E. et al.. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J Biol Chem. 270, 20615–20620 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

