Treatment of inflammatory arthritis via targeting of tristetraprolin, a master regulator of pro-inflammatory gene expression
- PMID: 27597652
- PMCID: PMC5446007
- DOI: 10.1136/annrheumdis-2016-209424
Treatment of inflammatory arthritis via targeting of tristetraprolin, a master regulator of pro-inflammatory gene expression
Abstract
Objectives: Tristetraprolin (TTP), a negative regulator of many pro-inflammatory genes, is strongly expressed in rheumatoid synovial cells. The mitogen-activated protein kinase (MAPK) p38 pathway mediates the inactivation of TTP via phosphorylation of two serine residues. We wished to test the hypothesis that these phosphorylations contribute to the development of inflammatory arthritis, and that, conversely, joint inflammation may be inhibited by promoting the dephosphorylation and activation of TTP.
Methods: The expression of TTP and its relationship with MAPK p38 activity were examined in non-inflamed and rheumatoid arthritis (RA) synovial tissue. Experimental arthritis was induced in a genetically modified mouse strain, in which endogenous TTP cannot be phosphorylated and inactivated. In vitro and in vivo experiments were performed to test anti-inflammatory effects of compounds that activate the protein phosphatase 2A (PP2A) and promote dephosphorylation of TTP.
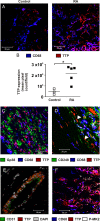
Results: TTP expression was significantly higher in RA than non-inflamed synovium, detected in macrophages, vascular endothelial cells and some fibroblasts and co-localised with MAPK p38 activation. Substitution of TTP phosphorylation sites conferred dramatic protection against inflammatory arthritis in mice. Two distinct PP2A agonists also reduced inflammation and prevented bone erosion. In vitro anti-inflammatory effects of PP2A agonism were mediated by TTP activation.
Conclusions: The phosphorylation state of TTP is a critical determinant of inflammatory responses, and a tractable target for novel anti-inflammatory treatments.
Keywords: Cytokines; Fibroblasts; Inflammation; Rheumatoid Arthritis; TNF-alpha.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures
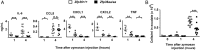




Comment in
-
Rheumatoid arthritis: Tipping the balance towards resolution.Nat Rev Rheumatol. 2016 Nov;12(11):622. doi: 10.1038/nrrheum.2016.159. Epub 2016 Sep 22. Nat Rev Rheumatol. 2016. PMID: 27652507 No abstract available.
-
PP2A plays a key role in inflammation and cancer through tristetraprolin activation.Ann Rheum Dis. 2017 May;76(5):e11. doi: 10.1136/annrheumdis-2016-210684. Epub 2016 Nov 3. Ann Rheum Dis. 2017. PMID: 27811146 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

