Key Targets for Multi-Target Ligands Designed to Combat Neurodegeneration
- PMID: 27597816
- PMCID: PMC4992697
- DOI: 10.3389/fnins.2016.00375
Key Targets for Multi-Target Ligands Designed to Combat Neurodegeneration
Abstract
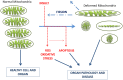
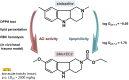
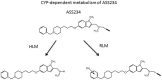
HIGHLIGHTS Compounds that interact with multiple targets but minimally with the cytochrome P450 system (CYP) address the many factors leading to neurodegeneration.Acetyl- and Butyryl-cholineEsterases (AChE, BChE) and Monoamine Oxidases A/B (MAO A, MAO B) are targets for Multi-Target Designed Ligands (MTDL).ASS234 is an irreversible inhibitor of MAO A >MAO B and has micromolar potency against the cholinesterases.ASS234 is a poor CYP substrate in human liver, yielding the depropargylated metabolite.SMe1EC2, a stobadine derivative, showed high radical scavenging property, in vitro and in vivo giving protection in head trauma and diabetic damage of endothelium.Control of mitochondrial function and morphology by manipulating fission and fusion is emerging as a target area for therapeutic strategies to decrease the pathological outcome of neurodegenerative diseases. Growing evidence supports the view that neurodegenerative diseases have multiple and common mechanisms in their aetiologies. These multifactorial aspects have changed the broadly common assumption that selective drugs are superior to "dirty drugs" for use in therapy. This drives the research in studies of novel compounds that might have multiple action mechanisms. In neurodegeneration, loss of neuronal signaling is a major cause of the symptoms, so preservation of neurotransmitters by inhibiting the breakdown enzymes is a first approach. Acetylcholinesterase (AChE) inhibitors are the drugs preferentially used in AD and that one of these, rivastigmine, is licensed also for PD. Several studies have shown that monoamine oxidase (MAO) B, located mainly in glial cells, increases with age and is elevated in Alzheimer (AD) and Parkinson's Disease's (PD). Deprenyl, a MAO B inhibitor, significantly delays the initiation of levodopa treatment in PD patients. These indications underline that AChE and MAO are considered a necessary part of multi-target designed ligands (MTDL). However, both of these targets are simply symptomatic treatment so if new drugs are to prevent degeneration rather than compensate for loss of neurotransmitters, then oxidative stress and mitochondrial events must also be targeted. MAO inhibitors can protect neurons from apoptosis by mechanisms unrelated to enzyme inhibition. Understanding the involvement of MAO and other proteins in the induction and regulation of the apoptosis in mitochondria will aid progress toward strategies to prevent the loss of neurons. In general, the oxidative stress observed both in PD and AD indicate that antioxidant properties are a desirable part of MTDL molecules. After two or more properties are incorporated into one molecule, the passage from a lead compound to a therapeutic tool is strictly linked to its pharmacokinetic and toxicity. In this context the interaction of any new molecules with cytochrome P450 and other xenobiotic metabolic processes is a crucial point. The present review covers the biochemistry of enzymes targeted in the design of drugs against neurodegeneration and the cytochrome P450-dependent metabolism of MTDLs.
Keywords: cytochrome P450; mitochondria; monoamine oxidase; multi target designed ligands; neurodegeneration; oxidative stress.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

