Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster
- PMID: 27598132
- PMCID: PMC5037727
- DOI: 10.3390/ijms17091448
Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster
Abstract
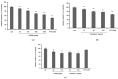
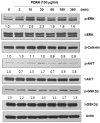
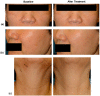
Polydeoxyribonucleotide (PDRN), a deoxyribonucleotide polymer, is popularly used for faster healing of cutaneous wounds and boosting of neocollagenesis of photoaged skin among current dermatologic practitioners. Some patients receiving PDRN injection treatment also reported improvement of photoaging-associated mottled pigmentation (PMP). To investigate the effect of PDRN on cutaneous melanogenesis, we examined the effect of PDRN and an available product (Placentex(®)) containing PDRN on melanogenesis using human melanocytes-keratinocytes cocultures and mouse melanocytes. Melanin content, tyrosinase activity, and levels of microphthalmia-associated transcription factor (MITF), tyrosinase, and tyrosinase-related protein (TRP-1) were determined. Intracellular signaling pathways were assessed by Western blotting. PDRN and Placentex(®) led to decreases in melanin content, tyrosinase activity, and MITF and TRP-1 expression with concomitant increases in phosphorylated forms of extracellular signal-regulated protein kinase (ERK) and AKT in mouse melanocytes. More importantly, both PDRN and Placentex(®) significantly suppressed the melanin content in human melanocyte-keratinocyte cocultures. Clinical evaluation of six female patients with facial hyperpigmentation after three sessions of intradermal PDRN injections using a 5-point scale revealed that PDRN led to more than noticeable improvements in hyperpigmented lesions. This is the first study to demonstrate that PDRN, which is known for its wound-healing properties, may have novel anti-melanogenesis and potential skin whitening properties.
Keywords: coculture; hyperpigmentation; melanogenesis; polydeoxyribonucleotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Squadrito F., Bitto A., Altavilla D., Arcoraci V., de Caridi G., de Feo M.E., Corrao S., Pallio G., Sterrantino C., Minutoli L., et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2014;99:E746–E753. doi: 10.1210/jc.2013-3569. - DOI - PubMed
-
- Galeano M., Bitto A., Altavilla D., Minutoli L., Polito F., Calo M., Lo Cascio P., Stagno d'Alcontres F., Squadrito F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16:208–217. doi: 10.1111/j.1524-475X.2008.00361.x. - DOI - PubMed
-
- Bitto A., Polito F., Irrera N., D'Ascola A., Avenoso A., Nastasi G., Campo G.M., Micali A., Bagnato G., Minutoli L., et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011;63:3364–3371. doi: 10.1002/art.30538. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

