SCN10A Mutation in a Patient with Erythromelalgia Enhances C-Fiber Activity Dependent Slowing
- PMID: 27598514
- PMCID: PMC5012686
- DOI: 10.1371/journal.pone.0161789
SCN10A Mutation in a Patient with Erythromelalgia Enhances C-Fiber Activity Dependent Slowing
Abstract
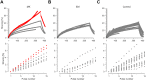
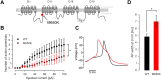
Gain-of-function mutations in the tetrodotoxin (TTX) sensitive voltage-gated sodium channel (Nav) Nav1.7 have been identified as a key mechanism underlying chronic pain in inherited erythromelalgia. Mutations in TTX resistant channels, such as Nav1.8 or Nav1.9, were recently connected with inherited chronic pain syndromes. Here, we investigated the effects of the p.M650K mutation in Nav1.8 in a 53 year old patient with erythromelalgia by microneurography and patch-clamp techniques. Recordings of the patient's peripheral nerve fibers showed increased activity dependent slowing (ADS) in CMi and less spontaneous firing compared to a control group of erythromelalgia patients without Nav mutations. To evaluate the impact of the p.M650K mutation on neuronal firing and channel gating, we performed current and voltage-clamp recordings on transfected sensory neurons (DRGs) and neuroblastoma cells. The p.M650K mutation shifted steady-state fast inactivation of Nav1.8 to more hyperpolarized potentials and did not significantly alter any other tested gating behaviors. The AP half-width was significantly broader and the stimulated action potential firing rate was reduced for M650K transfected DRGs compared to WT. We discuss the potential link between enhanced steady state fast inactivation, broader action potential width and the potential physiological consequences.
Conflict of interest statement
AMR, ZZ, HS, DM, PW, JK were employees of AstraZeneca (Sweden) at the time of the study. THC is a current employee of AstraZeneca (UK) and owns shares in AstraZeneca. This did not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

